





 - ah, no - the secret was that the
board is part of Polverones plan for world domination....
- ah, no - the secret was that the
board is part of Polverones plan for world domination.... 
| Quote: |




| Quote: |













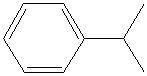
 pretty annoying writing and absolutely heavy and undigestible (maybe you can read
that for hours but in my case, I prefer to write half developped structures... it is more synthetic and visual than IUPAC names. Most chem books
display such writings (only the chem catalogue write the borring text...and as soon as they can use abreviated names or structural drawings).
pretty annoying writing and absolutely heavy and undigestible (maybe you can read
that for hours but in my case, I prefer to write half developped structures... it is more synthetic and visual than IUPAC names. Most chem books
display such writings (only the chem catalogue write the borring text...and as soon as they can use abreviated names or structural drawings).










 )
)





