
fluorescence - 12-2-2017 at 09:52
Hey guys,
So I am currently studying for an exam on asymmetric synthesis and catalysis and we had different methods vor selective allylations of aldehydes. The
so called Type II Reagents, often Tin or Silicon allyls can be used as either (E)-configured or (Z)-configured tin-allyl. Both of them yield
syn-products only.
Now for the Type I reactions you have a closed, cyclic, Zimmerna-Traxler transition state and it doesn't really matter if there is an (Z) or (E) allyl
as the repulsion isn't too big. So Z -> Syn and E-> Anti. For Type III like Cr(II)-Allyls the metallotropic shift from (Z) to (E) is so fast
that only (E) reacts so only the anti-product is formed.
Type II however does not have a cyclic state because the tin-reagents have to be activated using an additional lewis acid. You can write this in the
newman-projection (picture added below).
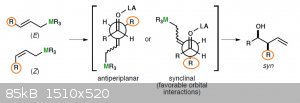
The synclinal state is further stabilized by an interaction of the Sn-C-bonding bond donation e-density into the C=O-antibond of the Carbonyl-group.
The problem I have here is why are both these states now syn ? What I am looking at is the "R" in the background which could be either syn or anti to
the C=O (OH) and in one case it's close to it while in the other case it is on the other side.
I'm not sure how an anti-product would look like in a newman projection but I just can't imagine the synclinal to have the "R" later on the same side
as the CO (OH). Do I look at this in a wrong way or how would I have to imagine an anti-product in this case ?
