
Boffis - 9-8-2017 at 15:04
Thiourea and benzyl chloride react to give S-benzyl-isothiuronium chloride, an important base used to characterize carboxylic acid and similar organic
compounds. The preparation is widely available eg in Vogel's Textbook of Practical Organic Chemistry and on the Org Synth web site. It is a monovalent
base.
Question is could I react a compound such as 1,2-dibromoethane or 1,3-dibromopropane with two moles of thiourea to obtain a bis(thiuronium bromide) a
divalent base? Many simple alkyl halide such as ethyl bromide react in this way and the procedure was once used to derivatise organic halides.
An extension of this is the reaction of activated aromatic halides eg 2,4-dinitro-chlorobenzene, would this give the appropriate
2,4-dinitrophenyl-isothiuronium chloride or a messy mixture?
Note: I have just noticed in Arun Sethi's organic chemsitry book he discribes the preparation of the bis thiuronium salt from 1,2-dichloroethane.
[Edited on 10-8-2017 by Boffis]
[Edited on 10-8-2017 by Boffis]
Cryolite. - 10-8-2017 at 17:10
Interesting. It just so happens that I have ~25 ml of benzyl chloride that I need to get rid of. Does anyone know of a good procedure to isomerize
ammonium thiocyanate into thiourea? If so, I'll give this a go.
Geocachmaster - 10-8-2017 at 17:40
"50 g of ammonium thiocyanate are melted in a round flask in a paraffin-bath, and kept at a temperature at which the mass remains just liquid
(140-145° C) for 5-6 hours. The cooled melt is powdered and ground with half its weight of cold water, which dissolves unchanged ammonium
thiocyanate, but little of the thiourea. By dissolving the residue in a little hot water, pure thiourea is obtained, on cooling, in colorless, silky
needles. The yield of thiourea is 7-8 grams. Thiourea forms colorless, rhombic prisms (from dilute aqueous solution), long silky needles (from
concentrated solutions); m. p. 172° C."
Low yield (15%) but I assume that the extra ammonium thiocyanate can be recovered.
Source: http://www.prepchem.com/synthesis-of-thiourea/
Edit: it would be much easier to hydrolyze the benzyl chloride with NaOH solution and recover benzyl alcohol if all you wish is to dispose of it.
[Edited on 8/11/2017 by Geocachmaster]
Boffis - 11-8-2017 at 13:43
I have just tried the reaction of thiourea (2 mole equ) with 1,2-dichloroethane (1 mole equ). To my surprise the reaction worked exactly as discribed
in Arun Sethi's book. I used 30g thiourea and 16ml of 1,2-dichloroethane in 140ml of white industrial methylated spirit. The thiourea was heated with
the methylated spirit until it dissolved and then the dichloroethane was added over about 3-4 minute. There was a vigorous reaction causing the
mixture to boil. The resulting solution was slightly cloudy so I added a pinch of decolorizing charcoal and filtered it hot through a 5.5cm buchner
funnel. The flask had to be heated to redissolve the crystal that formed before it could be poured into a basin to crystallise. The result was a good
crop of brilliant white crystals. The mixture was chilled in the fridge for an hour and filtered, the crystals washed with a little methylated spirit
and dried to yield 20.317g. This is only about 41% yield so tomorrow I'll try distilling off about half of the solvent to obtain a 2nd crop. I will
also do a Mp determination.
An attempt with some benzyl chloride I have had for some time failed inspite of testing it beforehand. The BC boiled at 177-178, (text books 179C), it
smells aromatic between naphthalene and xylene but it has a painful effect on the muscous membrains if you smell it closely and it fumes very slight.
[Edited on 11-8-2017 by Boffis]
Just a note: I have found out what is wrong with many of the preparations of Benzyl-isothiouronium chloride in Vogel and repeated elsewhere; they use
too much water to dissolve the thiourea. I have discovered that the resulting solution simply doesn't crystallize as described because the thiouronium
chloride is very soluble in water. In fact I evaporated a couple of ml to dryness on a water bath and then scaped the solid into the reaction mixture
(chilled to 4 C) and it dissolved showing the solution is nowhere near saturated. It is much less soluble in cold ethanol so the following preparation
is both simpler and easier.
Add 15.2g of thiourea (0.2 moles) to 50ml of 95% ethanol (rectified spirit) and add 25.3g (0.2 moles) of beor x fnzyl chloride. Warm until the benzyl
chloride dissappears (very rapid 30 seconds) and then reflux for 40-45 minutes and cool, remove a few drops onto a small watchglass and dry. Pour the
remainder into a basin, when at room temperature chill in the fridge and scrape the dried material onto the liquid and stirr gently. Crystallisation
occurs fairly rapidly and the whole mass solidifies. Stirr up with a spatula and filter at the pump, stuck dry with strong suction, turn out the cake
and dry. The filtrate should be evaporated slowly to a small volume and chilled to recover a second crop. At 1 mole and larger quantities it might be
worth recovering the solvent but at this scale is isn't worth it.
Would you like some pictures?
[Edited on 12-8-2017 by Boffis]
Boffis - 4-9-2017 at 05:18
I have now done several more experiments into the condensation of thiourea with alkyl halides and made a few interesting observation.
1) The crystalline material recovered from the reaction 1,2-dichloroethane and 2 meq of thiourea is just unreacted thiourea (hence the apparent 41%
yield) as determined by its melting point. In other words even on prolonged refluxing there is little or no reaction. However, the 1,2-dibromoethane
reacts fairly readily producing an easily crystallisable colourless compound. This reaction is actually described on the Org Synth web site. It is
hydrolized by alkalis to the fairly pungent ethanedithiol.
2) 1,4-dibromobutane reacts similarly but when I attempted to recrystallise the compound from iso-propanol it dissolved easily at first (about 2.5ml
per gram) but as the solvent began to boil colourless crystals that will not redissolve formed rapidly and within 30 seconds it appears that
practically all of the compound had precipitated in this form. Investigations continue.
3) Chloroacetic acid and thiourea. I didn't really expect much here but they do seem to react in aqueous solution on warming for a few minute and when
cooled the product crystallises in amazing 2cm long white prisms in good yield. The nature of this product is not certain but I believe that it is the
isothiuronium acetic acid chloride because of its high solubility in water.
 Crystals of isothiuroniumacetic acid chloride?
Crystals of isothiuroniumacetic acid chloride?
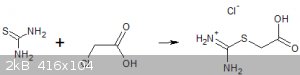
When sodium chloroacetate is used instead of chloroacetic acid a completely different reaction occurs and a sparingly soluble compound is formed that
I believe is 2-amino-1,3-thiazolidone.
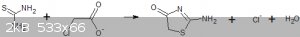
I am going to test the acetic acid derivative to see if I can hydrolysed to thioglycollic acid or whether it cyclotizes to the thiazolidone
derivative. I am going to test the second compound to see if it can be diazotised or nitrosated.
One of my interests in the acetic acid derivative is to see if it can be reacted with hydrazine solution to give aminoguanidine and diaminoguanidine
in the same way that ethyl thiuronium bromide can but producing the none volatile thioglycollate ion instead of the rather smelly ethanethiol.
Boffis - 1-11-2017 at 09:56
I have continued to dig into this interesting and useful group of organic salts and discovered that some aromatic versions do exist. Quite by accident
I stumbled across this paper in the old volumes of the Journal of the Chemical Society in which they tried reacting 2 and 4-nitrochlorobenzene,
2,4-dinitrochlorobenzenene, 2-nitro-1,4-dichlorobenzene and picryl chloride with thiourea (called thiocarbamide in the paper). Only
2,4-dinitrochlorobenzene proved reactive enough to give the isothiuronium salt. Though Picryl chloride reacted two to give various product.
Taylor and Dixon; chloronitrobenzenes and thiocarbamides; JCS 1924; v125, p243
Does anyone have any suggestions as to the identity of the white prismatic compound above? Could it be a simple chloroacetate salt of thiourea? Is
thiourea a strong enough base to crystallise with an acid of the strength of chloroacetic acid (pKa 2.85)? Any idea how I can test the two
possibilities? I wondered if I could try forming a sparingly soluble salt of a bulky monovalent anion like sodium tetraphenylboron or a
tetraiodobismuthate salt and then see what is left in the aqueous phase chloride or chloroacetate ions.
Boffis - 11-1-2020 at 10:34
Once again while browsing through old journals I have stumbled across an interesting little gem. The reaction between chloroacetic acid and thiourea
described above has been worked on before and the product identified as "formamidinethiolacetic acid" as I suspected, though I gave it a different
name. When the reaction is carried out in water the product is reportedly free of HCl but when the reaction is carried out in acetone the product
crystallises as the hydrochloride.
Ray & Fernandez; Action of monochloroacetic acid on thiocarbamide (thiourea); JCS, v105, p2159 [1914]
