
chemrox - 6-2-2007 at 12:17
I am looking for a writeup on making propionyl chloride from propionic acid and thionyl chloride. I'll wing it from other acid chlorode writeups if I
have to but would like to use an established regimen if someone has it. All I know now is the ratio of agent to acid is 2:1 to avoid anhydride.
That would seem to mean putting them all together at the outset or adding the acid to the agent. A drying tube would seem appropriate. Not sure
about gas traps.
chemrox - 6-2-2007 at 12:56
Vogels uses an example in which 0.5 mols are used as 47g !!??
(119g/mol x 0.5 mol = 59.5 g x ml/1.5 (20deg) = 39.7ml)
Sauron - 6-2-2007 at 13:52
First of all, thionyl chloride is not the only choice for this kind of transformation.
Secondly the use of excess reagent is for driving the equilibrium to the right, and is rtather wasteful of a hard to obtain reagent that is difficult
to prepare.
There are many threads here about other chlorinating reagents for making acyl chlorides and acyl anhydrides.
I'd suggest using the search engine.
Several of us have posted articles with general experimental details for more than one reagent, and details on how to prepare one of the most useful
reagents.
Finally, it seems I didn't need to tell you how to make propionic acid, or did you buy it?
Search on:
chlorination
acid chloride
anhydride
There are two or three reagents to find, apart from the ones Vogel cites. All of the ones he mentions are now difficult to obtain in many parts of the
world due to CWC restrictions. Those are thionyl chloride, phosphorus trichloride and phosphorus oxychloride. But just move on to the alternatives.
The alternatives are:
Oxalyl chloride
Cyanuric chloride (CC) also called TCT or trichloro-s-triazine
The first is rather expensive, but it can be made from the second.
The second is an inexpensive solid.
Do not confuse CC/TCT with trichloroisocyanuric acid which is closely related and also useful.
not_important - 7-2-2007 at 00:34
What about sulfuryl chloride, from SO2 and Cl2? While it's used as a chlorinating agent, I remember seeing references to its use for making acid
chlorides. Or does it give too much in the way of side products?
Sauron - 7-2-2007 at 02:14
Normallr if you want to go this route, per an old article cited on Rhodium, you use it to make anhydrides not acyl chlorides.
And you make sulfuryl halide in situ. So, sulfur and Br2 get you your reagent, anhydrous sodium acetate is added and a strange semisolid semiliquid
form, bromine color disappears. After distillation under reduced pressure Ac2O in high yield is redistiller through a fractionating column at
atmospheric pressure. It's a Russian prep Chemistry & Industry in the 40s IIRC.
While you could do that with chlorine it would be less convenient.
And I regard either as pretty inferior to the use of CC/TCT with or without DMF. Unless you have a lot of Br2 to spare and who does? I pay $100 a Kg
for mine I think CC is a lot cheaper than that.
Bromine and sulfuryl chloride are both dangerous and unpleasant to work with. CC is a solid, it's pungent but much milder than those.
You could also go the longer way round the block and make oxalyl chloride with CC, then use it to make either the acyl chloride or the anhydride as
you desire.
But better to save oxalyl chloride, which is just as nasty as Br2 to work with, for those special occasions when nothing else will do.
Like making POCl3 from P4O10 or (maybe) PCl3 from phosphorous acid (excess reagent dehydrating it to P2O3 in situ). Or AsCl3 from the oxide. Verboten
chemicals not easily accessible any other ay.
Oxalyl chloride can be purchased but it is not cheap. As CC is cheap and so is oxalic acid or anhydrous sodium oxalate, making it is more economical.
garage chemist - 7-2-2007 at 02:25
Br2 and S do not make sulfuryl bromide, but sulfur dibromide instead. I assume it was a typo.
Instead of sulfur and bromine, you can use sulfur dichloride or disulfur dichloride which are both cheap and easy (though NOT pleasant) to make by
chlorination of sulfur.
I did the preparation of Ac2O from Br2, sulfur and NaOAc. It didnt work well. The product was seriously impure as seen from the distillation curve,
and also had a funny smell probably from brominated derivatives.
$100 for 1 kg of bromine? What do you pay for a kg of NaBr or KBr? It would surely be cheaper to make it yourself.
I once paid 80€ for 1 Liter (not kg) of bromine (I have only a fraction of it left). That was not too expensive. But the prices have risen
considerably until now. I would have to calculate if it is cheaper to make than to buy it.
Sauron - 7-2-2007 at 02:46
Yes I know what S and Br2 gives you on paper. I am reporting what the Russian piece cited on The Hive/Rhodium claimed was the mechanism. How they got
from S and Br2 to SO2Br2 is a mystery to me, and I have not tried this prep to see if it works, since I found far better ways to get to Acetyl
Chloride and Acetic Anhydride.
I'd guess either SBr2/S2Br2 oxidized readily or that these were the brominating agent and not sulfuryl bromide as claimed. Russian error, translation
error or Hive error? Dunno. I have it here somewhere in hardcopy and when I find it I'll scan and upload.
The most irritating thing about this prep was that it called for some Ac2O in the starting mixture. Now what is the point of requiring some product to
make the same product?
I found this complete archive of the several procedures including the one I had in mind on Erowid, attached bwlow. The error regarding drying temp has
not been removed. All reference to SO2X2 (X=Cl, Br) has been removed. Instead the active agent is given as suflur chloride or bromide. So either my
memory is bad or someone revised this. <shrug> But I specifically also recall mention of the stirred mix being a peculiar semisolid semiliquid
that seemed to slidify when stirring was interrupted then liquified again when stirring resumed. That vivid description stuck in my mind, but does not
appear in the text below, so I think on Rhodium there was more discussion of this procedure and maybe that's where the SO2Br2 mechanism was advanced.
------------
"Acetic Anhydride and Propionic Anhydride
Drone #342:
Here's what everyone's looking for. Some things are a little wierd about it, like the fact in the acetic anhydride synth they use a small quantity of
acetic anhydride as a solvent. However, as one sees in propionic anhydride, such a solvent may not be necessary.
From "Chemistry & Industry", 1945, p. 382; "LABORATORY METHOD FOR THE PREPARATION OF ORGANIC ACID ANHYDRIDES" by Jehuda Orshansky and Eliahu
Bograchov.
"...(1) Acetic anhydride. To 50 g. acetic anhydride in a round-bottomed flask of 1500 cc. capacity, placed in cold water, 440 g. of powdered sodium
acetate (dried by fusion at 320 C) and at the same time a solution of 22. g of sulfur in 320 g. bromine is added while stirring. The operation takes
about 30 minutes.
"The mixture is then stirred for a further 5 minutes, after which period the initially dark brownish-red colour has changed into pale yellow, and the
anhydride is distilled off from a water bath under reduced pressure. The crude anhydrie (310 g) is redistilled under normal pressure, and the fraction
boiling between 134-138 C is collected. Yield, 295 g. of 98% purity = 87.5%. The so purified anhydride contains neither bromine nor sulphur compounds
and leaves no residue on evaporation..."
"(2) Propionic anhydride. To 40 g. fused and powdered sodium propionate in a flask of 250 cc. capacity a solution of 2 g. sulphur in 22 g. bromine was
added while stirring. The temperature was kept at about 50 C. When the operation was completed, the anhydride was distilled off in vacuo. The crude
product (25g.) was fractioned under normal pressure, and the fraction 155-156 C was collected. Yield, 23 g. propionic anhdride of 90% purity = 85%..."
The paper mentions that other alkali metals and alkali earth metals work just fine. Calcium propionate is a food preservative added to cheap white
bread to keep it from molding. With these nuggets of information, the most closely watched reagent on the DEA's watched list, propionic anhydride,
just turned OTC. I can almost see the fentanyl analogs clogging the opioid market already.
The one reason, and justafiably so, they poo-poo using chlorine (which does indeed work nicely) is that its a hassle to work with, especially
considering the fact that they'd be adding a gas to a solid to make a liquid. I propose that perhaps with the use of chloroform as a solvent, chlorine
could be bubbled in readily, and the reaction would go as previously stated. I assume they tried to avoid using extra solvents in hopes of staying
away from azeotropes messing up their products' purity, so this may or may not work, depending on what you're trying to do."
----------------
Bromine: Actually I paid $75 for 1.5 Kg (500 ml). Too many middlemen. Now that I have found the local agent for Carlo Erba I can probably get it for
$50. I think that is 33 cents a gram. They limit me to a couple bottles a month, but since they shouldn't be selling it at all (it's a MOD-restricted
item for which I should need a license) I don't complain as I doubt my requirements will ever get near 36 Kg a year.
I do use NaBr to replace HBr aq in bromination of some alcohols/glycols.
[Edited on 7-2-2007 by Sauron]
[Edited on 7-2-2007 by Sauron]
[Edited on 7-2-2007 by Sauron]
[Edited on 7-2-2007 by Sauron]
Attachment: http___www.erowid.org_archive_rhodium_chemistry_anhydrides.pdf (34kB)
This file has been downloaded 2622 times
Sauron - 7-2-2007 at 03:13
Oh, and @chemrox:
Vogel teaches that the SOCl2 methos cannot be applied to acetic and propionic acids because the bp's of the resulting chlorides are too close to that
of the excess SOCl2 to permit seperation by fractionation.
He advises that thionyl chloride be used for acid chloriced of normal C4 acids and higher.
So, take my advice and use CC instead.
Now, if you can't buy propionic acid or a propioniate salt, and elect not to oxidize 1-propanol for some reason, all is not lost.
Buy a opropionate ester like methyl or ethyl propionate and saponify it.
Often the esters of even watched acids are readily available (cf., phenylacetate esters, anthanilate esters).
But cheapest/easiest ought to be oxidation of n-propanol and I'd expect a wide variety of oxiders to work. See Vogel and take your pick.
[Edited on 8-2-2007 by Sauron]
Thanks one and all!
chemrox - 8-2-2007 at 20:58
What a thread! you'd think all the uses of this common reagent were suspect! I'm working with fuels! Anyway, what I got, also from Vogels, is the
two have close boiling points but excess SOCl2 is destroyed with formic acid which can be separated by fractional distillation. I already have the
SOCl2. And as someone pointed out, the examples in Vogels do not use a great excess. Also, less environmental concern and fewer side reax. But what
a wealth of information this generated.
Sauron - 8-2-2007 at 21:19
What environmental concerns might there be with CC? The byproduct is solid cyanuric acid, and that is useful in and of itself (you can chlorinate it
to trichloroisocyanuric acid for example.)
SOCl2 generates SO2 (as in acid rain) and HCl while oxall chloride, CO and CO2 (greenhouse gases) but I would say the two are about equal on that
score.
However since you already have the stuff, do as you please. I suspect that if you employed a H-scavenger like Pyr or TEA, you would not need any
excess of SOCl2 at all, and the resulting amine hydrochloride would be solid amd insoluble in your rxn mix, just filter off and wash. The point of the
excess reagent was to drive rxn to the right against competition from HCl being formed. The H-scavenger would do same thing, snap up the acid as it is
made. The SO2 will not trouble you, it will offgas.
Reference Information
solo - 8-2-2007 at 21:24
A Convenient Preparation of Volatile Acid Chlorides
HERBERTC . BROWN
Journal of the American Chemical Society 1938, vol:60 iss:6 pg:1325
Summary
A simple general procedure for the preparation of volatile acid clorides is described, involving the distillation of the desired acid chloride from a
mixture of benzoyl chloride and an organic acid. The procedure has been used in the prepara- tion of twelve aliphatic acid chlorides with yield of
70-90%. The mechanism of the reaction is discussed. NO definite condusions concerning the mechanism could be reached.
Attachment: A Convenient Preparation of Volatile Acid Chlorides .pdf (439kB)
This file has been downloaded 3481 times
Sauron - 8-2-2007 at 22:20
Thanks @solo. Another tool in the arsenal.
Time for a cost analysis and comparative study of the facility of this vs TCT vs oxalyl chloride and for those who can still get it, thionyl chloride.
The last is not an option for me.
garage chemist - 9-2-2007 at 01:26
Benzoyl chloride is a way better choice than thionyl chloride for preparation of volatile acid chlorides like acetyl and propionyl chlorides.
Organikum specially mentions that thionyl chloride is not suitable for the preparation of those due to high losses with the SO2 and HCl offgas and
high residual SOCl2 content of the acid chlorides.
Benzoyl chloride can be made from benzotrichloride and benzoic acid with ZnCl2 as catalyst. I once opened a thread about this here and a patent for
this process had been posted there.
One can even make acetyl chloride directly from benzotrichloride and acetic acid with ZnCl2.
Benzotrichloride is made by chlorination of toluene at reflux and under strong light.
Sauron - 9-2-2007 at 02:45
You can also chlorinate benzoic acid or sodium benzoate to the benzoyl chloride with TCT alone or TCT/DMF.
Not toat it does people much good where TCT is hard to obtain.
Except, see the thread on CS2 + Cl2 and find out how to make CC from Cl2 and MeSCN. Along with other useful products.
If you can buy or make a methyl halide and buy KSCN or NaSCN you can make MeSCN easily.
Sauron - 9-2-2007 at 03:41
Acros sells benzoyl chloride and benzoic acid for roughly same price. And it's rather cheap.
I'll see what a 25 Kg sack of the technical benzoic acid goes for here. Then figure the cost of making a Kg of benzoyl chloride from that and TCT/DMF.
Then compare that to the landed cost of the Acros benzoyl chloride which from experience is about 2X the catalog price. That would be in vicinity of
$40-$45 US a Kg.
Then compare the cost of using TCT directly to make propionyl chloride to the cost of doing same with benzoyl chloride.
TCT does have 3 active chlorines, benzoyl chloride only one, and that will tend to make a difference in molar rations and thus cost.
I'm not a TCT salesman and have no particular ax to grind, or as say, no dog in this fight. I'm as interested in finding the best routes to acid
chlorides and anhydrides as anyone else.
Maya - 9-2-2007 at 06:03
What is the difference between Cyanuric chloride (CC) also called TCT or trichloro-s-triazine and trichloro-s-triazinetrione?
I have tens of kilo's of the latter............
Nicodem - 9-2-2007 at 06:07
"Trichloro-s-triazinetrione" is just a bad name for trichloroisocyanuric acid.
Maya - 9-2-2007 at 06:10
I see
1,3,5-Trichloro-2,4,6-trioxohexahydro-s-triazine; 1,3,5-Trichloro-s-triazine-2,4,6(1H,3H,5H)-trione; 1,3,5-Trichloro-s-triazinetrione;
1,3,5-Trichloroisocyanuric acid; Isocyanuric chloride; Symclosene; Trichlorocyanuric acid; Trichloroisocyanuric acid;
1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-trichloro-; s-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-trichloro-
Sauron - 9-2-2007 at 08:09
Two different but related compounds with very different properties.
Cyanuric chloride is trichloro-s-triazine, the trimer of cyanogen chloride. The chlorines are attached to carbons.
Trichloroisocyanuric acid, in contrast, has the chlorines on the nitrogens.
CC is used industrially to manufacture agricultural pesticides.
Trichloroisocyanuric acid is mainly used as a swimming pool chlorinator.
CC One of my favorite molecules because it is so useful. Acid chlorides from acids, acid anhydrides therefore by extension. Alkyl chlorides from
alcohols. In concert with KBr, alkyl bromides from alcohols.
To give credit where it is due I first learned of this one on Rhodium maybe 5 years ago.
The other I am becoming more fond of, I only encountered it two months ago here. Live and learn.
[Edited on 9-2-2007 by Sauron]
[Edited on 9-2-2007 by Sauron]
Maya - 9-2-2007 at 08:25
Thats what I thought,
so trichloro-s-triazine is not the same as trichloro-s-triazinetrione
or
Cyanuric chloride is not the same as Trichloroisocyanuric.
For a minute there I thought my pool chemicals had other uses ( which they do just not this )
Sauron - 9-2-2007 at 08:29
[108-77-0]
C3Cl3N3
[Edited on 9-2-2007 by Sauron]
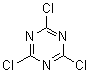
Sauron - 9-2-2007 at 08:42
[87-90-1]
C3Cl3N3O3
[Edited on 9-2-2007 by Sauron]
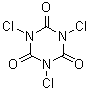
chemrox - 9-2-2007 at 08:57
I found benzoyl Cl to be cheaper than TCT. Thanks vey much. Looks like the SOCl2 method gives shitty yields. The acid is cheap the SOCl2 is not and
best be saved for higher weight acids.
Sauron - 9-2-2007 at 09:32
TCT is about $2 a Kg cheaper, but has 3 active chlorines to benzoyl chloride's 1, and does not have to be used in excess. Stoichiometric proportions
are fine. Normally you figure only two of the chlorines will be used, so to chlorinate a monobasic acid you'd use 1 mol TCT to 2 mols of acid. 1:2 not
2:1.
With benzoyl chloride you are advised per that JACS article to use a 50% to 100% excess (3:2 or 2:1) on molar basis of the benzoyl chloride vs the
acid substrate.
Half a mol TCT to do one mol of acid, versus 1.5 to 2 mols benzoyl chloride to do same.
That is 3-4 X more reagent to do same job on same acid and, does not bode well for the cost effectiveness of benzoyl chloride vs TCT.
Harder to quantify are matter of ease of workup, etc.
At that point it becomes a matter of personal preference.
Acros sells CC (TCT) for $30 a Kg, and benzoyl chloride for $27 a liter. d1.2 so that liter is 1200 g. Still...3-4 to one.
chemrox - 9-2-2007 at 22:48
TCT looks pretty attractive. What is the workup?
Sauron - 10-2-2007 at 00:03
We've posted quite a number of different articles on the forum in different threads, use Search and download the files pls.
However I can summarize the different TCT to acyl chloride processes as follows:
1. (old) applicable to acetic acid and other liquid acids.
Relux TCT with a large excess of acid until no more insoluble cyanuric acid precipitates. Filter off the cyanuric acid byproduct and wash with some of
starting acid. Combine washing with filtrate and distill the acid chloride from the unreacted starting acid.
2. (old) Dry distill sodium salt of acid with small excess of CC.
3. To a solution of the acid TEA in acetone add CC in acetone and stir at RT 4 hours. Cyanuric acid ppts out. Filter. What remains is a mixture of
acid chloride, unreacted acid, and TEA in acetone. Rotavap off the acetone bubble in dry HCl to ppt out the TEA as hydrochloride, filter, distill. See
article for molar ratios.
4. Form TCT/DMF comples, react this with acid. See article for workup.
The first two procedures are perhaps best for larger scale preps.
The third prep is designed for convenience especially if you want to use the acyl chloride in situ, many examples are given of this.
The fourth is very efficient and high yielding.
How much propionyl chloride do you ant to make?
Here's a zip file, there's the main TCT/DMF article, plus a Brazilian review, and a JACS piece on using TCT (CC) to make alkyl chlorides from
alcohols.
I am still hunting the Tett.Lett article on CC in acetone w/TEA.
[Edited on 10-2-2007 by Sauron]
Attachment: TCT_DMF.zip (589kB)
This file has been downloaded 1136 times
Sauron - 10-2-2007 at 01:56
@chemrox, the reference you are still lacking (after downloading above attachment) is Tett.Lett. 40 4395 (1999), author M.Faloomi et al.
Organikum - 15-2-2007 at 11:28
The industrial process for making alpha-chloropropionate starts from lactic acid + thionylchloride and the Ullmann says yields are a baffling 100%.
Chlorination of propionic acid is NOT trivial, old literature says that PCl3 is difficult and workup almost impossible.
I think that there is reason why industry starts with lactic acid and its not only the price......
Knowing that thionylchloride is hard to impossible to come by and PCl3 the same I remember having read something about chlorination with TCCA + a
small amount of PCl3 as "starter". A small amount of PCl3 can be produced without bigger problems (some P - can be even scraped from matchboxes - in
chloroform or DCM + dry chlorine gas should nicely produce PCl3, it makes SnCl4 and AlCl3 why not PCl3....). TCCA is easy and lactic acid should be
not so big a problem (getting it anhydrous might be a challenge though).
And where is now this benzaldehyde, oh! forgot! First we have to make some ester of this alpha-chloropropionate - well anhydrous alcohol and dry
HCl-gas should do the trick here or not? 
garage chemist - 15-2-2007 at 11:37
Red P in Chloroform + Chlorine I have done on a medium scale (10g red P in 100ml Chloroform, the Chloroform was previously dried over P2O5 and
distilled).
This exclusively produces phosphorus pentachloride. No PCl3 is present in the mix, even when red P is present in excess.
The PCl5 just refuses to react with more red P when in Chloroform (in the dry state it works, however).
http://www.versuchschemie.de/ptopic,124916,2d7537946855c29dc...
Organikum - 15-2-2007 at 12:14
PCl5? Even better. 
Das "von mir erfunden" ist aber ein wenig arg mutig oder nicht?
Danke & Nix für ungut!
/ORG
Sauron - 15-2-2007 at 12:27
I must have missed a post. We are discussing making pripionyl chloride not a-chloroprionic acid.
SOCl2 and PCl3 are reagents for acyl chlorides not alpha chlorination.
In Hell-Volhard-Zelinsky rxn you might use a catalytic amount of PCl3 or red P as halogen carrier but you could just as well use pyridine or sulfur or
SCl2 in same fashion and with similar results.
N-chlorosuccinide in CCl4 on the acid chloride is a specific a-chlorination reagent.
Likewise NBS to make the alpha-bromoacid chloride which is then converted back to the a-bromo acid. I use that one to make a-bromocaproic acid on my
way to norleucine. At least when I get tired of HVZ using up my Br2.
For acetic and proprionic acids, SOCl2 or PCl3 are problematic in workup for sure because of bp's too proximate (as Vogel advises) but again they'd be
making acid chloride not chloro acid. They work for C4 acids and higher. But I still say they have been made obsolete at least on lab scale by TCT,
TCT/DMF, benzoyl chloride, and oxalyl chloride. These work fine on acetic and propionic acids too.
[Edited on 15-2-2007 by Sauron]
[Edited on 15-2-2007 by Sauron]
Organikum - 15-2-2007 at 12:50
My bad. I apologize. But somehow this all sounded exactly like making alpha-chloropropionate, but I should have looked harder.
Pardon
/ORG
chemrox - 16-2-2007 at 22:45
sure is nasty stuff ... I tried a drying tube at the end of the 500mm west condenser .. wrong termination .. after being expelled from the lab (gotta
fix that hood fan) I ran a gas tube through a backflow trap and down the sink with the cooling water. Now i'v got a pot full of benzoic acid
leu - 18-2-2007 at 14:50
If the yield is sufficient, this approach would probably be the most facile for most  Mssr Colson published this synthesis of propionyl chloride from propionic acid, acetonitrile and HCL gas in Comptes Rendus
121 1155-6 (1895)
Mssr Colson published this synthesis of propionyl chloride from propionic acid, acetonitrile and HCL gas in Comptes Rendus
121 1155-6 (1895) 
Attachment: colson.zip (30kB)
This file has been downloaded 1037 times
john_do - 31-5-2007 at 05:32
How does one go about getting this text translated into english?
Sauron - 31-5-2007 at 08:46
@Leu, I downloaded that file and tried to open it with WinZip and got an error message saying it is not a valid zip file
WinRAR opened it but all that appeared was gibberish code.
Can you post this in unzipped form?
S.C. Wack - 31-5-2007 at 13:25
The problem is at your end again. Nothing wrong with it with winrar.
For something on-topic to say, an excerpt from JACS 44, 1746 (1922) that will not help the thread starter but the thread is here, so...
To 540 g. of propionic acid 245 g. of sulfur monochloride was added and chlorine
was passed through the mixture while it was stirred for 15 hours. The chlorination
proved to be incomplete at this point, since 150 g. of unchanged propionic acid was
recovered. There was also considerable sulfur monochloride, which was removed from
the propionyl chloride by treatment with hydrogen sulfide. The propionyl chloride obtained
amounted to 250 g.
not_important - 31-5-2007 at 20:50
On a WinDoze XP machine the OS unzipped the file fine, as did 7zip (free, multiplatform) under Windows and Linux.
There's been problems for various people with downloading some files recently. I do not think it is the original files, but some problem during
download.
Here is the unzipped form, reads just find with Irfanview + djv plugin. Compression saved about 1% it looks like.
And I just tried D/L on the attached, or more properly viewing in FireFox with the djv viewer plugin, and it went OK.
[Edited on 1-6-2007 by not_important]
Attachment: colson.djvu (30kB)
This file has been downloaded 783 times
not_important - 1-6-2007 at 01:16
With XP right-clicking on the file and selecting "Open with..." will let you select the built-in WinDoz zip function.
I've now been told that the zip file in questions was successfully processed by registered versions of Winzip 10.something and 11.1
If there are problems opening compressed attachments in the future, hopefully one of the options in the last few messages will solve the problems.
Polverone - 1-6-2007 at 07:40
I've removed the most off-topic junk from this thread. If people want to keep talking about software and file attachments, make a thread elsewhere.
chemrox - 22-12-2007 at 18:05
which is this TCT or CC or something else? It's called trichlorocynuric acid and it oxidizes sulfites to sulfoxides which can be useful
Attachment: RSR to RSOR by TCT.pdf (44kB)
This file has been downloaded 1413 times
Sauron - 22-12-2007 at 19:58
The paper title says trichloroISOcyanuric acid.
That is TCCA Not TCT/CC which are the same thing (TCT = CC)
We have several times posted the structures and proper nomenclature for both os these are are disinclined to do so again.
Suffice it to say that with TCCA the three chlorines are on nitrogen, it is a cyclic tri-chloramine commonly used as swimming pool chlorinator.
TCT (CC) is the acid chloride of cyanuric acid. The chlorines are on carbon, replacing -OH groups.
The empirical formulas are different, the molecular weights are different, the structural formulas are different. Why do people keep confusing these
two? Chemrox old friend, go sit in the corner facing the wall and wearing a pointy hat.
TCT contains no oxygen. It is C3N3Cl3.
TCCA is C3N3O3Cl3.
Their chemical behavior is very different as you might expect.
chemrox - 22-12-2007 at 22:42
@Sauron -It sure does say "..iso.." that was careless. Thank you.
Sauron - 22-12-2007 at 22:50
No problem, even I have been wrong, once.
Klute - 23-12-2007 at 13:09
Translation of Comptes Rendus, 121 1155-6 (1895)
ORGANIC CHEMISTRY- Synthesis of amide hydrochlorides and acid chlorides.
Note of Mr. Albert COLSON, presented by Mr. P. Schützenberger.
"On contact with hydrochloric acid, a lot of amides only fix one molecule of acid for two molecules of amide, and no general processes that only give
the stoechiometric combination of the two compounds are known. We present herein a process that simultanously give monohydrochlorides of amides and
acid chlorides, with great ease and high yields. After having mixed a nitrile with an acid, the mixture is saturated with dry gaseous hydrochloric
acid at 0°C. The reaction is the following:
RCN + R'COOH + 2HCl = RCONH2.HCl + R'COCl.
Examples: According to this equation, the following experiments are conducted:
1°- A stoechiometric mixture of acetonitrile CH3CN and acetic acid was saturated with dry hydrogen chloride at 0°C and left in a
sealed flask for a day. A large amount of acetamide monohydrochloride crystals CH3CONH2.HCl precipated; at the same time a corresponding amount of
acetyl chloride was formed.
2°- When replacing the acetic acid in this experiment by proprionic acid, the same hydrochloride was obtained aswell as an
equivalent amount of propionyl chloride.
3°- By saturating with dry hydrogen chloride some propionitrile CH3CH2CN dissolved in cooled acetic acid, a abondant amount of
propioamide monohydrochloride crystals CH3CH2CONH2.HCl were obtained the next day, aswell as some acetyl chloride.
4°- Formic acid, of which the chloride is unknown, decomposes itself much more readibly than its homologues: it gives carbon
monoxyde aswell as the hydrochloride of the nitrile employed.
The described process is thus a simple and practical way of obtaining acid chlorides without the use of dangerous phosphorus chloride, aswell as the
monohydrochloride of a amide.
Besides, the reaction on which this process is based on gives a new view on the esterification of alcohols by acides. We know that this operation is
usually done in presence of hydrogen chloride gas, and that the esterification of alcohols by acid chlorides is quantitative. The very efficient role
of hydrochloric acid is thus explained by the ease with which this gas converts organic acids into acid chlorides when a secondary compound removes
the water that is formed:
CH3COOH + HCl = CH3COCl + H2O
In the previous reactions, it's the nitrile that removes the water; during the esterification, it's another portion of the hydrochloric acid that
gives HCl +2H2O.
Furthermore, the amount of heat generated is comparable in the two cases.
Other process: The formation of acid chlorides is easier when the acid is replaced by it's anhydride. This was seen with acetic
anhydride:
(CH3CO)2O + 3HCl + CH3CN = 2CH3COCl + CH3CONH2.HCl
Thus, the explanation I gave about the formation of lactic diamides (Comptes Rendus, Dec 1895, p.825) must be modified. We
must consider the intermediate formation of an acid chloride that then reacts on the monoamide initially formed.
It's precisely when trying to control in simple experiments the explanation given in my last communication at the Academy that I discovered the
results of this present note. I thus suggest to generalize this method."
Sauron - 24-12-2007 at 04:53
Is this a suggested route to acetyl chloride?
Convenient if one has a cylinder of anhydrous HCl around.
Or a solution of HCl gas in AcOH. That is commercially available. At a rather high molarity if I recall.
Klute - 24-12-2007 at 10:04
It's the translation of the french article Leu linked further up the thread.. Acetonitrile is not that expensive, and acetamide can be usefull for
many things, including as solvant. HCl can be easily generated, although at a relative large scale it produces alot of waste (dilute H2SO4, which
could be used to neutralize basic wastes). Could be worth a try, if someone was desperate for an acyl chloride. I guess benzoic acid could work too,
why not oxalic or phtalic acid?
HAs anyone ever stumbled on similar refs? I'd be willing to translate french ones.
propionyl chloride experiment using TCT
chemrox - 29-1-2008 at 23:47
At the suggestion of a member and one relying on memory I tried a synthesis of propionyl chloride using TCT by two different pocedures. I'm reporting
what I did here for the good of the order.
Pocedure I
1 mol, 184 g. TCT was combined with 10 moles, ~1000 ml, propionic acid in a flask fitted with a column, head and condenser. The receiver was set with
a drying tube and acid vapor exhaust line. The mixture was stirred and heated to reflux. It was held at reflux for a total of 10 hrs over two days.
About 20 ml of propionyl chloride and an unknown low boiling compound were collected (acetyl chloride? from where?)
The reaction mixture was cooled collected and the phases separated: unreacted TCT and propionic acid. The latter was distilled the following day
whereupon 800 ml acid were collected in a highly pure state.
Procedure II
1/2 mol TCT, 82 g was dissolved in the minimum, 600 ml, amount of technical acetone. 140 ml propionic acid was added and the mixture stirred in a
beaker. It was planned to add 138 ml of Et3N but the first few ml reacted vigorously and the addition was stopped while everything was transferred to
a reaction/distillation setup as above. The rest of the base was added over an hour. The reaction was mildly exothermic and produced a white
precipitate.
The mixtures will be re-evaluated in the morning. I think my brain slipped a gear while I was smoking on the porch and writing the numbers in my
notebook. If any remedial adjustments seem indicated they will be made in the morning prior to the first phase separation. The mixture was *supposed
to be* 1/2 mol: 1 mol: 1 mol. Obviously there's excess acid and base.
I plan to decant the liquid and filter the residue. The collected liquid will be flashed to get the acetone off and then distilled to collect the
propionyl cl. The white ppt is not Et3N-propionate. I checked by test tube exp. It should be DCT according to theory. I can check later by IR.
Post script :
I edited to explain a stoichiometric foible- I put basic info for each ingredient in my notebook and used the bp instead of the weight for the acid so
it's there in excess. Shouldn't make a difference but there it is..
[Edited on 30-1-2008 by chemrox]
scale-up of anhydride prep failed
chemrox - 4-4-2008 at 22:19
I wanted to make about 200 ml of prop anhydride and used the fused Na salt method. This was an example of scale-up not working. I ended up with a
lot of unreacted Na-prop, Br2 and some damp Na-prop in the 3L flask. The stirrer had to be manipulated by hand, barely moved the material around and
mixed it insufficiently. Eventually the stirrer blade became disconnected. Unpleasant experience. Br vapor in the eyes etc.. The one bright spot ws
my partner located a used eyewash station and we're picking it up next week and installing it asap. Will use the prop-Cl/Na-prop method next.
tiger1 - 14-4-2008 at 15:35
The JACS 60 (6) 1325 1938 for propionyl cl with benzoyl chloride:propionic acid 3:2 is simple and it's very smooth. Simply charge both to RB flask
w/mag. stirring, heat to bp of 80 and you get a relatively pure fraction which if refractionated using a vigreux gives 80-90% or higher if you're
especially careful. Quick and clean. You can even recover the benzoic acid if desired with little effort. PCl5 also works well but is nasty to deal
with and is relatively expensive.
Sauron - 14-4-2008 at 19:09
That is the H.C.Brown article, I believe. Chemrox is well aware of it. It's a good procedure as long as the target acyl chloride is volatile enough
and propionyl chloride certainly is.
I was going to use this method to prepare chloroacetyl chloride, and still might. I have the chloroacetic acid and benzoyl chloride. But I think I
will try the GAA + TCCA method to make chloroacetic acid and then use TCT to get to the chloride. Save the benzoyl chloride for something more
interesting.
ChemPlayer_ - 8-7-2015 at 01:06
I tried the Comptes Rendus, 121 1155-6 (1895) method using HCl gas bubbling through chilled acetonitrile and glacial acetic acid.
The gas dissolved in the liquid very readily to the point that I ran out of salt and so didn't even get to see where saturation point was, even using
25% more than the theoretical amount (i.e. 2 x molar equivalents + 25%).
Left overnight the resulting liquid generated pretty snowflake crystals and on distillation the fuming liquid that was decanted off yielded a small
amount (8% based on acetic acid) of acetyl chloride.
So this works but needs some playing around to get a better yield (my first attempt at the reaction so I assume the bad yield is my inexperience
rather than the reaction itself at this stage). Definitely has potential I think as a non-CWC route to acyl chlorides...
The usual video shows the process I followed: https://www.youtube.com/watch?v=Q2ArDw0vf2Q
Magpie - 8-7-2015 at 08:33
Your video is, as usual, very well presented. Have you seen my similar method?:
http://www.sciencemadness.org/talk/viewthread.php?tid=32991#...
Who owns the sexy female voice of the narrator in your videos? She has such a steady, almost metered, delivery. All her words have a nice lilting
inflection, being almost musical. 
ChemPlayer_ - 9-7-2015 at 21:16
Yes I saw that - very nice!
It seems relatively easy to convert anhydride to chloride and chloride to anhdride (heat with sodium acetate and then fractionally distill the product
- we tried this and it works ok), but not so easy to get the chloride / anhydride in the first place from other sources without using the usual highly
restricted reagents.
We're also having a play with the sodium pyrosulfate route to the anhydride although actually making even vaguely pure pyrosulfate is extremely
difficult even with accurate temperature control. If this has any success then we'll create a video on this too, but at the moment our instinct is
that it won't be viable.
Why that is Tess, our corporate spokeswoman 
Magpie - 10-7-2015 at 09:24
acetic anhydride is a tricky one. It is currently available from the secondary market in the US. However, I understand many countries have it banned
or restricted.
Making it using sulfur halides is feasible for the home chemist. See my procedure in Prepublication. The key to making this a facile procedure is to
use some of the anhydride as a solvent, I believe. So once you have some on hand making it becomes much easier.
Hi Tess!
byko3y - 10-7-2015 at 10:25
Why can't you just use anhydrous acetic acid as initial solvent? Contamination of anhydride with acetic acid is completely acceptable.
ChemPlayer_ - 12-7-2015 at 00:15
Magpie, your method intrigues me and makes me wonder if it's possible to produce some aromatic acid chlorides in the same way.
I wonder if phthalic anhydride would react with HCl gas (perhaps molten, or in a solvent system) to produce an acid chloride, or perhaps even a 'half
chloride' (I guess this might convert into the full chloride and phthalic acid).
I will do some experiments with this and see...



 Mssr Colson published this synthesis of propionyl chloride from propionic acid, acetonitrile and HCL gas in Comptes Rendus
121 1155-6 (1895)
Mssr Colson published this synthesis of propionyl chloride from propionic acid, acetonitrile and HCL gas in Comptes Rendus
121 1155-6 (1895) 

