
JJay - 7-10-2017 at 10:31
I looked around but didn't see a general thread on organic nomenclature, but if there is one, this probably belongs there.
I noticed recently that chemists seem to use different ordering rules for styrene than for other chemicals. The discussion on Ask Dr. Shulgin seems to
confirm this, but he is beating a strawman here: http://www.cognitiveliberty.org/shulgin/adsarchive/nomenclat...
I'm reluctant to dismiss Shulgin's statements as the rantings of a lunatic amphetamine chemist, but I'm not really seeing a lot of hard and fast rules
on prioritization of the alpha carbon in academic discussion of the topic: http://www.chem.ucla.edu/~harding/IGOC/A/alpha_carbon.html
Indeed, industry seems to use similar nomenclature: https://www.alfa.com/en/catalog/L03609/
What exactly are the rules on prioritization of the alpha and beta carbon?
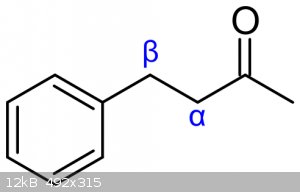
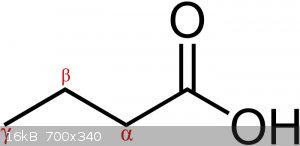
(Images found on Wikipedia.)
unionised - 7-10-2017 at 11:18
Alpha is the next carbon along, beta is the one next to that.
The problem is where do you start.
In ring systems you (sometimes) start at the ring, and in ketones you start at the carbonyl.
It's usually apparent from context.
JJay - 7-10-2017 at 12:07
If you treat styrene as an alkene, things are a little more complicated than if you treat it as a ring system: https://socratic.org/questions/how-do-you-name-alkenes-and-a...
Melgar - 7-10-2017 at 15:07
I guess you'd name styrene derivatives similar to, say, toluene derivatives. After all, the "-ene" at the end is used for aromatic rings, because
technically they're alkenes as well.
JJay - 7-10-2017 at 15:15
Eh, alkenes don't have the same kind of resonance as aromatics, although there are some similarities. But come to think of it, styrene would have
resonance that isn't like just another alkene too....
Melgar - 7-10-2017 at 19:37
Well, yeah. The point is that alpha, beta, etc. go away from the "main" or "defining" functional group. You can usually tell what the main
functional group is by looking at the suffix. However, structures like benzene, toluene, ethylbenzene, p-xylene, etc. have the benzene ring as their
main functional group. You'll notice that these all end in "-ene" too. Styrene is the same type of case, except that it also contains an aliphatic
alkene functional group. That doesn't really matter though, because its defining group is still the benzene ring. There's just confusion because
"-ene" is a suffix for both alkenes and benzene derivatives, I guess. But it still follows the pattern of all the other benzene-ring-containing
substituted hydrocarbons that end in "-ene".
JJay - 7-10-2017 at 21:19
Yeah, I'm actually not sure how much it matters since it's a terminal alkene, but that resonance does explain why it is so easy to isomerize terminal
phenylpropenes to beta-methylstyrenes. Or I think so anyway. Science demands an answer 

