
Metacelsus - 17-3-2018 at 12:34
I am currently trying to prepare a compound of the type shown below:
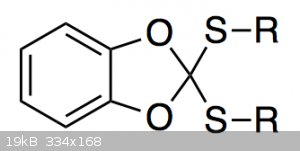
I can make it just fine, but purifying it is annoying because it slowly decomposes in contact with silica gel. The main decomposition product is
catechol carbonate.
I've heard adding 1% triethylamine to the eluent (which is 10% ethyl acetate in hexanes) might help. Alternatively, switching to alumina instead of
silica could work. However, I don't have alumina TLC plates so I can't do TLC using alumina. Distillation and crystallization are out of the question,
since the compound is a liquid that is also sensitive to heat.
Does anyone here have experience with purifying acid-sensitive compounds by chromatography? What do you think I should do?
[Edited on 3-17-2018 by Metacelsus]
DraconicAcid - 17-3-2018 at 13:19
I haven't done a lot of chromatography, but I suspect that alumina is generally more acidic than silica. Maybe calcium carbonate would work?
Metacelsus - 17-3-2018 at 13:55
I was thinking "basic alumina, Brockman grade I." For example: https://www.sigmaaldrich.com/catalog/product/sigald/199443 Note that it says the pH of a 5% suspension in water is 9.5
Judging by periodic trends, I would expect aluminum oxide to be less acidic than silicon oxide, all else equal. However, the acidity of alumina vs.
silica gel would probably depend on the method of preparation.
I haven't ever heard of calcium carbonate being used as a stationary phase, but I will look into it.
byko3y - 17-3-2018 at 18:03
Why haven't you already try to add triethylamine to the eluent? That's the first thing I think about, and you mentioned this option first too.
