
h0lx - 4-5-2007 at 08:22
I came across this in bed last night thinking of chemistry(geeky, I know)
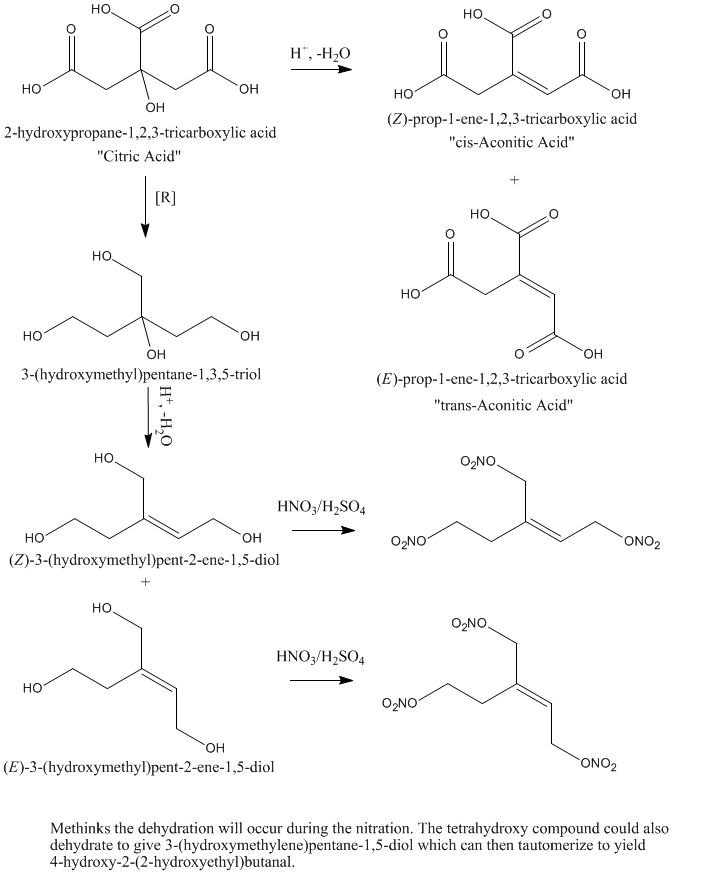
What if one would take citric acid and reduce it with a strong reducting agent like LiAlH4 to yield something I would call(sorry for errors)
1,3,5-hydroxy 3-hydroxymethylpentane and then nitrate it to get 1,3,5-nitro 3-nitropentane. This would be probably similiar to PETN in properties.
I need to go through all my chem suppliers to see if they have LiAlH4, I would try it out.
EDIT: Made a crappy paint drawing of it.
[Edited on 4-5-2007 by h0lx]
EDIT2: New better image, thank you Ramiel.

[Edited on 5-5-2007 by h0lx]
DrP - 4-5-2007 at 08:30
The middle molecule looks like pentaerythritol.
Sauron - 4-5-2007 at 08:40
I don't think that tertiary nitro ester looks very stable.
The_Davster - 4-5-2007 at 17:54
Looks viable. Only a couple additional methylene groups when compared with PETN. Be sure to look up some standard procedures for reducing carboxylic
acids to alcohols, would be a shame to waste LiAlH4.
Lithium Aluminum Hydride
MadHatter - 4-5-2007 at 18:29
Also be aware that LiAlH4 is a watched chemical on the DEA's drug precursor list
if you live in the U.S. any quite probably most places in the world. It may not be worth
the hassle.
Ozone - 4-5-2007 at 18:51
That would work..Except... You would probably be nitrating hydroxyl aconitic acid analogue; the tertiary hydroxyl is prone to dehydrate...
Aconitic acid is the same molecule minus the 3° hydroxyl plus a double bond in either the cis or trans position (trans predominates under
thermodynamic control).
Hmm. Run it cold and slow and it might be do-able (at least with some yield), but that hydroxyl will likely dehydrate if you look at it wrong.
hmm,
O3
[Edited on 5-5-2007 by Ozone]
Ramiel - 4-5-2007 at 19:06
Citric acid has also been the subject of fantasy for me for many cold nights, the fact that it has so many carboxyl groups is immediately seductive. I
also noticed that it has a fairly flexible structure, given a bit of prompting, I bet it could undergo some very interesting polymerization reactions.
For instance, I found it was a shame that peroxides are so unstable. Given the tendency for such a simple peroxide as acetone peroxide to form di- and
tetramers, surely 2-hydroxypropane-1,2,3-tricarbaldehyde would form some fascinating polymeric, crystalline and (relatively) stable peroxides?
Feel free to use http://www.sciencemadness.org/scipics/an_idea1.gif
vulture - 5-5-2007 at 10:41
You're going to waste ALOT of LiAlH4 if you're stupid enough to reduce citric acid directly. Not to mention the danger involved.

