Sodium borohydride reduction of nitrostyrenes by Reverse addition: A simple and efficient method for the large-scale preparation of phenylnitroethanes.
Synthesis, 1985, 9, 886-887
SWIM has been fucking around with reducing nitrostyrenes to nitroethanes and also got moderate yields, but now when using this paper, yields are great


 ). Peer-reviewed research chemists can be wrong. Patent chemists can be wrong, and even prone to mislead.
). Peer-reviewed research chemists can be wrong. Patent chemists can be wrong, and even prone to mislead.


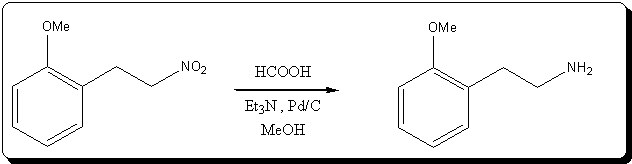




 ), so it was not triethylammonium
chloride (mp= ~240°C) [Note 5]
), so it was not triethylammonium
chloride (mp= ~240°C) [Note 5]