Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
T-butanol from Citric acid?
Could t butanol be made from citric acid by first making dimethyl citrate, followed by the complete reduction of the open carboxcilic acid, followed
by decarboxcilation?
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
What reducing agent do you plan to use to reduce a carboxylic acid to an alkane, or alcohol then alkane? Also, looking at the structure, you'd
probably want to form the symmetrical monoester, and decarboxylate twice, followed by reduction to the local alkane.
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Would esterification of those two groups cause them to be selectively decarboxilated? Or are you sugesting to form the symetrical ester, followed by
reduction to the alcohol, followed by decarboxlyation, followed by reduction of the alcohol to the alkane?
Edit:
I have a route in mind. Form the dimethyl ester, then decarboxylate. Reduce the remaining carboxcylic acid to an alcohol using NaBH4 . Then tosylate
the alcohol. Reduce to the alkane using NaBH4 through nucleophilic substitution.
[Edited on 4/8/2019 by Jackson]
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Sodium borohydride does not reduce carboxylic acid groups. However, it can reduce the acid to the alcohol if you first form the methyl ester.
[Edited on 8-4-2019 by DavidJR]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
But how would you selectively esterify a specific carboxyl instead of making a mixture of random esters?
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
i remember seeing somewhere that the different carboxcilic acid groups have different pKa so they could react in a certain order
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
I know pKa indicate how complete the acid dissociates into H+. Does it really have anything to do with esterification equilibrium?
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
As I understand it, the proposed route looks something like this:
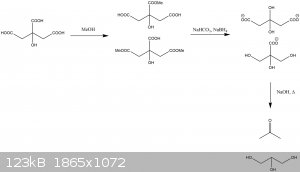
I have used a combination of sodium bicarbonate and sodium borohydride to neutralise the free acid groups without hydrolysing the ester and to reduce
the consumption of borohydride by protonation.
Edit: Picture error
[Edited on 9-4-2019 by 12thealchemist]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
@12ta some of the C in backbone is missing
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
You are quite right.
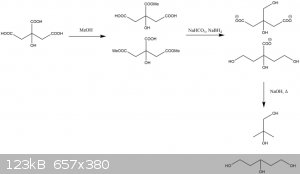
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
If you're looking for an OTC source of t-butanol, consider instead the dietary supplement hydroxymethylbutyrate, which is just a single
decarboxylation away from t-butanol.
http://en.wikipedia.org/wiki/3-hydroxy-3-methylbutyrate
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Was looking at the ingredients on the back of a can of lynx Africa the other day and it says contains tert butyl alcohol.citric acid seems like a long
way away to start from.
|
|
|