teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Sodium pyroantimonate preparation
Today I tried a nice synthesis of sodium pyroantimonate. I found the procedure in Schlessinger, "Inorganic laboratory preparations" (preparation 49).
I just wanted to do something with antimony and test my H2S generator upgrade. The synthesis consists of 4 stages:
1) Sb2O3 + 6HCl = SbCl3 + 3H2O
2) 2SbCl3 + 3H2S = Sb2S3 + 6HCl
3) Sb2S3 + 6NaOH
Actually I found different products of the last reaction. Schlessinger gives
Na2SbS3, Na3SbO3 and H2O, but Treadweel gives NaSbS2, NaSbOS and H2O. (Also could be because really Sb2S3 has many different forms).
4) (according to Schessinger)
Na2SbS3 + 4NaOH + H2O2 = NaSb(OH)6 + 3Na2S
Na2SbO3 + H2O2 + 3H2O = NaSb(OH)6 + 2NaOH
The resulting pyroantimonate is not similar to pyrophosphate, it is just a historical name for the compound which structure was initially discovered
as Na2H2Sb2O7 * 5 H2O but really it contains octahedral oxygen complex ion Sb(OH)6- which P and As doesn't form for some reason. So, pyroantimonate is
quite unique compound of antimony. (see Wells, "Structural Inorganic Chemistry").
I started the synthesis by dissolving Sb2O3 (from a pottery shop) in HCl. It is mandatory to use concentrated HCl because in 2M HCl Sb2O3 will not
completely dissolve. After dissolving the solution was diluted by water to have it 2M in HCl. This is minimal concentration when insoluble in water
oxychloride can be redissolved with help of little shaking (any drop of water into HCl/SbCl3 mix gives white SbOCl which can be dissolved again if the
concentration is greater than 2M). Also, possible this is a maximal concentration when H2S can precipitate Sb2S3 (see Treadwell).
Actually I did 2 tries and the first was a failure (due to some errors I did in H2S generator). So, for the second try I used some "cloudy" solution
of SbCl3 (with SbOCl) which was less than 2M in HCl. After passing the stream of H2S this solution becomes yellow (see the picture 1)
and then even more intense colour of Sb2S3 appeared (picture 2).
I stopped generator not waiting till all Sb2S3 will precipitate (it was just a test run and I found that FeS in a form of a single lump is for very
patient people, the stream of H2S was just too slow).
I did vacuum filtering of Sb2S3 (mixed together with some SbOCl/Sb2O3) and then dissolved it in NaOH. Then I did vacuum filtering through a glass
filter to filter out SbOCl/Sb2O3 and got a clear filtrate (picture 4).
I did the rest by Shlessinger (dropping 6% H2O2 into 70-80 C solution) and got a clear white precipitate which I hope is NaSb(OH)6.
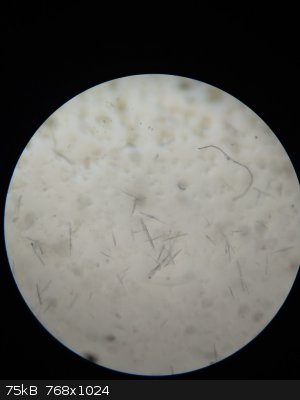
I checked the form of crystals with a microscope. The form is quite interesting - some of them looks like boxes which are empty inside but this effect
is due to refraction of light near crystal surface (picture 3).
I will check which experiments I can do with this compound. First, I should find a way to proof it is NaSb(OH)6. Possible with checking its
solubility. Then I have 2 ideas: proof it is Sb(V) and try to grow bigger crystals. It also interesting try it as an oxidizer. But most remarkable
property could be the refraction index (see the picture of the crystal).
  

[Edited on 9-9-2019 by teodor]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Interesting preparation, I wonder if it will work from black antimony sulphide from antimony and sulphur as this would be easier than H2S
precipitation of antimony sulphide. I remember making Schlipp's salt once from natural stibnite (boil stibnite, NaOH and sulphur), quite a lot of
white precipitate forms that has to be filtered off and is supposed to be a sodium antimonate.
I wonder if you could do the same with KOH? The potassium salt is much more water soluble and was once used as a gravimetric reagent for sodium, this
would be a useful and interesting compound to play with.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Agree, it is good to try this preparation with different forms of Sb2S3 and Sb2S2O also. I try to understand how many forms of Sb2S3/Sb2S2O exist, how
they differ chemically so now I am reading some books just to get idea how I can ever classify them. As woelen said in this thread he got Sb2S3 with different colours between yellow and orange and I've got a crimson modification of probably Sb2S2O.
But I think all of them should form Na[Sb(OH)6] and K[Sb(OH)6] because oxidation to Sb(V) should remove all the differences. But the differences
possible could be in different antimony acids we can get from different form of the the sulfide.
I followed Schlessinger method (with H2S) also because I tried to find answer whether is it possible to precipitate Sb2S3 from 2M HCl solution. The
problem is that with dilution you get SbOCl and with more acidic solution you get no precipitation of Sb2S3. So, I found that 2M of HCl is a proper
concentration and I need it for improve my analytical methods (I still use the old way of qualitative analysis of different substances with H2S).
Also I think Schlessinger wrote a method for Na[Sb(OH)6] because it could be easily precipitated from the solution so additional recrystallization etc
is not necessary.
I also have idea to get K[Sb(OH)6]. Today I tried to oxidize SbCl3 with K2Cr2O7 but it was chrome and antimony, compounds which have reach chemistry
of its own and now they meat each other, so I totally messed up with the results which I've got, I think I should plan this experiment better. But I
think I didn't get any K[Sb(OH)6] today by this method.
Surely I can try to oxidize some antimonate with H2O2. I will try to write something if I will have success. I appreciate any ideas, so please make
suggestions, how I can try to do it starting from SbCl3 or Sb2O3 as an antimony source. I mean by the easiest way,
[Edited on 12-9-2019 by teodor]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Oxidation with H2O2 certainly works. I used that to make a mix of Sb(III) and Sb(V) in conc. HCl in order to make the dark blue cesium salt of the
mixed oxidation state (III,V) chloro complex of antimony.
I can imagine that using both chromium and antimony leads to very complicated and ill-defined (or at least not investigated) complexes. Chromium(III)
is capable of coordinating to almost everything!
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
teodor - interesting reaction, thx.
As Boffis mentioned... I have some black Sb2S3 so I definitely must try this sometimes, using both hydroxides (Na, K).
teodor - growing bigger crystals... maybe using KOH and then adding Na+ slowly?
Also Li salts precipitate from K solution according this article
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Thanks Fery. Following by one of the references from the URLed article I found this work: https://www.researchgate.net/publication/237853019_Pyrochlor...
which lists all known methods of the preparation.
For me the simplest are these 2:
1. Oxidation of Sb2O3, in alkaline solution using KMnO4 or H2O2, followed by addition of alkali hydroxide.
2. Precipitation of the metal antimonate by adding the appropriate metal salt to the colloidal "antimonic acid".
@woelen
Never heard about cesium salt with mixed Sb valencies. Has cesium some specific?
Yes, you found very proper words to say what I think about my Sb + Cr experiment.
[Edited on 13-9-2019 by teodor]
[Edited on 13-9-2019 by teodor]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Potassium pyroantimonate preparation
As Boffis wrote
"I wonder if you could do the same with KOH? The potassium salt is much more water soluble and was once used as a gravimetric reagent for sodium, this
would be a useful and interesting compound to play with".
And I agree.
But I spent some time until I have managed the preparation of KSb(OH)6 * nH2.O.
I found the procedure in the old german paper - "Uber amphotere Oxydhydrate, deren wiRrige Losungen und kristallisierte Salze." Jander & Brull -
https://onlinelibrary.wiley.com/doi/abs/10.1002/zaac.1926158...
The preparation is very interesting, some details are very important and I did it twice to understand the procedure.
Some general information first. Sb2O3 is not easily soluble in an alkaline solution. I prepared syrupy solutions of NaOH, heated it to 100 C or
something like that (may be more), but 50 g of NaOH in this very concentrated form was failed to dissolve 3 g of Sb2O3 completely. So, for the
preparation of the antymonate slow addition of H2O2 is essential. It oxidize Sb(III) to Sb(V) and the Sb(V) compound (potassium pyroantimonate) is
very soluble in hot water.
Another important thing is a temperature. Boiling of KSb(OH)6 possible converts it to a salt of different antimonic acid and addition of a cold water
to the hot solution deposits oxides which is "not easy to dissolve again". Above 65C H2O2 starts to decompose. So, I tried to keep the reaction
mixture in 57 - 63 C temperature range.
Also, at the beginning the concentration of KOH is very high and hot mass dissolves a bit of glass, so to make pure compound (without much Si) the
addition of H2O2 should be continuous to finish the preparation as soon as possible. But really it could be completed not faster than in 30-40 mins.
Now the procedure.
1. Prepare a water bath which will allow to raise the solution temperature up to 60 C. Prepare a cold water bath. Prepare a stand which will allow the
solution to be immersed in the water bath and pulled out to keep the temperature near desired 60 degrees C.
2. Prepare 220 ml of 9% H2O2 solution. This concentration possible contains an optimal amount of water to dissolve the antimonate and to allow its
separation at the last stage. I used 2 portion - cold and warmed to 60 degrees. Most of the time I used the warmed solution.
3. Put 31 g if Sb2O3 and 23 g of KOH in 250 ml beaker,

put the beaker into cold water and add 10 ml of water inside the beaker. Stir the mass well. It becomes very hot. Protect yourself from hot fumes of
KOH during the whole procedure (I used a gas mask).
4. Now get a thermometer and put into the mass. Start addition of H2O2 drop by drop with constant stirring with the thermometer. Try to decrease the
temperature to 60 degrees. It should be possible after some amount of H2O2 is added and the thermal contact with the beaker walls is improved. When
drops of H2O2 are decomposing that means the solution is still too hot. So, do it slowly - one drop - stirring - next drop until you can reach 60
degrees.
5. Continue addition of H2O2 from a beaker drop by drop with constant stirring. After some point the concentration will reach the point when you will
need to put the mixture on a water bath. Don't stop addition of H2O2 just regulate its speed.
6. After all the peroxide is added the solution is milky-white due to very small bubbles of oxygen.

Keep it on the water bath at 70 degrees C until all H2O2 will be decomposed and all bubbles will disappear.

7. Filter it when still hot through a glass filter.

8. Put it into 500 ml beaker, allow to cool and add 200 ml of methanol.
 (the picture shows a cooling process) (the picture shows a cooling process)
9. Put it somewhere for at least 12 hours. The oily drops of antimonate will form a lower layer. Don't remove the methanol until you will be ready to
proceed further. Otherwise the mass will solidify.
10. After 48 hours the mass has a consistency of soft plastic.

Decant the liquid and put the mass piece by piece into mortar, stir it well with 50% methanol/water solution (by mass). It is very exothermic process,
not for the solution but for your body. Just stir it until the mass will become a powder and pour it, portion by portion, on a filter which is in a
funnel attached to a vacuum. After all the powder will be collected wash it 2-4 times with the same 50% methanol after that transfer for drying, but
separate from the filter first otherwise it sticks to it. UPD: it still colours an universal indicator paper showing an alkaline reaction of the wash
liquid after 4 washes, but not sure is it KOH absorption or the basic properties of the salt. I stopped washing and I think I will be able to correct
the pH with acid in my experiments later.

11. Wash everything with hot water.
The compound is really interesting. It dissolves very slowly in cold water but fast in a hot water (~50 C). Almost all metals except K gives insoluble
salts (so, you can get, for example, K salt having soluble Na salt) and the form of the crystals are different for different cations. When the
concentration is high you get the deposit immediately,

(reaction with Na+)
but with diluted solution you can get very nice crystalls.

(Sodium pyroantimonate crystalls on a test tube walls)
(Barium pyroantimonate crystalls on a test tube walls)
It reacts with thiosulfate in slightly acidic environment and forms some antimony sulfide.
Upon addition of acids it deposits antimonic acid.
.
[Edited on 24-9-2019 by teodor]
[Edited on 24-9-2019 by teodor]
[Edited on 24-9-2019 by teodor]
[Edited on 25-9-2019 by teodor]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nicely done theodor! And a good write-up too. I'll have to have a go at this procedure as I would like to play with this interesting compound.
I think a solution of the potassium salt will always react alkaline because the "antimonic acid" is a weak acid.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Thank you, Boffis.
Yes, you are right, but I forgot that when did washing. If I properly remember it is even weaker than carbonic acid. If it is so, does it mean CO2
could slowly react with this compound if it is not stored in a closed bottle?
I also like to do more experiments with this compound, for example, I am wondering how it will react with gaseous HCl. Will it form SbCl5, SbCl3,
SbOxCly, SbxOy or nothing will happen.
[Edited on 25-9-2019 by teodor]
[Edited on 25-9-2019 by teodor]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
That's an interesting point. Is hexahydroxyantimonic acid V a stronger acid than carbonic acid? I don't know but sodium hexahydroxyantimonate
(pyroantimonate) occurs naturally as the mineral mopungite so maybe but the natural mineral is very rare and may also be more stable than carbonates
under mildly acidic conditions. I have seen a paper somewhere about the stability of mopungite v brizziite NaSbO3 but I can't find it at present.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Oh, searching for "mopungite" in google led me to Burtite Ca[Sn(OH)6], Mushistonite (Cu,Zn,Fe)[Sn(OH)6], Natanite Fe[Sn(OH)6], Schoenfliesite
Mg[Sn(OH)6], Vismirnovite Zn[Sn(OH)6],Wickmanite Mn[Sn(OH)6], even Jeanbandyite with FeFe[Sn(OH)6]... (iron II and III) and Tetrawickmanite which has
really some weird formula. Probably they are minerals known to a very small group of people only. But they definitely are some stable compounds. It is
interesting also that the cation can be in different oxidation state .
I would like to know whether Fery's idea of slow addition of another salt to a diluted solution of KSb(OH)6 can give bigger crystalls? Will try to do
some experiments with this also. By the way, this photo of mopungite shows the same crystal structure as in my picture of NaSb(OH)6 deposit.
Also, no one mineral in this list: https://www.mindat.org/min-2777.html
contains both chromium and antimony. Is it not existing compound? Woelen already said something interesting about that, so it will be really
interesting to discover Sb and Cr mixed in some mineral in nature.
Also, if somebody will try to make K[Sb(OH)6] by this procedure could you please check the smell of the solution in the beginning of 8th step. My
solution had some really remarkable aroma which I hardly can associate with an inorganic compound. May be some impurities in my case?
[Edited on 25-9-2019 by teodor]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Some update to the procedure. It is better to seal K[Sb(OH)6] just after filtering - in any case it is hard to say how many molecules of water it will
keep as a water of crystallisation (and dehydration should be possible by heating according to literature). I kept my sample for a week on a filter
paper in a cupboard and it changes its property: it was reacted with air and now doesn't give clear solution. So, now I must filter the solution every
time. The portion which was sealed immediately doesn't give any precipitate. Other properties are the same.
In my case I also dried KHSO4 in the same cupboard, so not sure whether it was CO2 which changed the properties.
[Edited on 30-9-2019 by teodor]
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Interesting. This salt is useful because it is soluble. But im interested in if co, ni and cu react with this salt and produce pyroantimonate.
|
|
|