chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
methylation of nucleophilic HN- with methyl iodide?
always wondered about this.
i dont indend on doing this, but do you think that methyl iodide could possibly be able to methylate the primary amine in the plot below to a
secondary amine? and them maybe protonate the leftover iodide to HI? not too sure about the mechanism, but theoretically i think this could work.
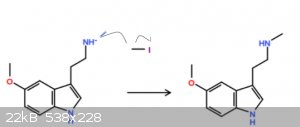
[Edited on 28-11-2019 by chemist1243]
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
It definitely can. However, you will get a mixture of products as:
It will not stop at the secondary amine, you will get some tertiary and possibly quaternary too. Using an exact stoichiometric amount of the
alkyl halide is not sufficient to avoid this unfortunately - you still end up with a mixture of products + unreacted starting material.
It will also methylate the indole nitrogen
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quote: Originally posted by DavidJR  | It definitely can. However, you will get a mixture of products as:
It will not stop at the secondary amine, you will get some tertiary and possibly quaternary too. Using an exact stoichiometric amount of the
alkyl halide is not sufficient to avoid this unfortunately - you still end up with a mixture of products + unreacted starting material.
It will also methylate the indole nitrogen
|
thats strange. you would think that with stoichiometric amounts there would simply not be another methyl group to methylate any thing else other than
the negatively charged nitrogen. are the other methyl groups derived from a different source, or am i not thinking about this the right way?
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Stoichiometric rations do not mitigate against side reactions.
Additionally, there are equilibria to consider. You need concentrations such that the reaction is driven forwards.
These are general principles in all organic chemistry. Actually not just organic. I am not commenting on his reaction in particular. I just don't
think you should be surprised.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quote: Originally posted by j_sum1  | Stoichiometric rations do not mitigate against side reactions.
Additionally, there are equilibria to consider. You need concentrations such that the reaction is driven forwards.
These are general principles in all organic chemistry. Actually not just organic. I am not commenting on his reaction in particular. I just don't
think you should be surprised. |
thats makes more sense. thank you.
|
|
|