vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Easy Extraction of Lactose and Casein from Milk
This notes will help to isolate both milk protein ( casein) and milk sugar ( lactose ) from milk
For this experiment I have used skimmed sweet milk.
For those who dont want to read the notes they can directly go into video which explains all of these
https://youtu.be/DBS5g4BoSZY
Materials required
Skimmed sweet cows milk - 100ml
1N HCl (or any other acid) - 10ml
Calcium carbonate - 4g
Activated Charcoal - 2g
Methanol/Ethanol - 100ml
Milk is a complex colloid containing a lot of substances like
Water,fat,casein,whey proteins,lactose and minerals.
Principle : Casein precipitated by acid
filtered off
Excess acid neutralised by calcium
carbonate
Solution boiled to precipitate out whey
proteins and then filtered.
Boil solution to make it 1/4 then add
charcol to decolourise
Then add alcohol ( since lactose is more
soluble in alcohol)
Procedure
Take 100ml of skimmed milk in a beaker. If you are using regular milk just remove the fat floating on top by heating the milk to boil .
Now add 10N HCl. While adding the acid there should be constant stirring ,only then the acid will penetrate down the precipitated casein,otherwise the
casein precipitated on top act as a barrier preventing the acid from reacting with the bottom layer.
Now filter the solution to get the precipitated casein out of the solution. I just used gravity filtration method. Vaccum filtration will be much
faster . Only casein has got precipitated here because it's the negatively charged molecule and it's charges get neutralised on adding the acid which
made it to seperate out from the colloid .
The clear solution obtained after filtration is called whey. It still contain some other proteins like lactalbumin and they are called as whey
protein.
Now we add the calcium carbonate to remove the acid .We did that because the acid present in it can decompose the lactose in the next step of heating
it.
To remove all the other soluble proteins we boil the solution so that the proteins get denatured and precipitate out from the colloid.
Now ultimately filter the solution and then boil the solution to about 1/4th .
The solution will now have some nasty dark color. We will add some activated charcoal and keep it for 30min so most of it will get decolourised.
Now we add the alcohol and mix it up well and filter to remove the charcoal and any other insoluble residue.
We now let the solution sit in direct sunlight so that alcohol will get vapourised and beautiful crystals lactose will get crystalised. Collect the
crystals ,dry them and dissolve them once again in little amount of alcohol and allow the alcohol to vapourise and recrystallise lactose. Weigh
lactose and the casein which we made in the initial part .
So that's pretty much it. Hope you like my notes
Now if you want, you can watch the video here
https://youtu.be/DBS5g4BoSZY
I have also added the picture of lactose that I crystallised out .

Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
nice experiment!
do you have a way to test the lactose? like mp for example
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Lactose can be tested with the usual test for carbohydrates
Molisch test to confirm the presence of sugar.
Then the Benedict's test to make sure it is a reducing sugar
Osazone test to confirm the lactosazone by microscopy
I have planned on a video of it soon
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
hi vibbzlab, well done !
in the video you used 100ml of methanol and you let it evaporate on the sun - was it outside?
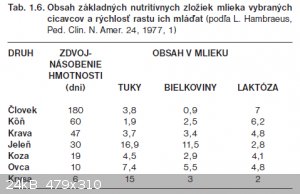
25g of dried casein from 100ml of milk, some HCl reacted with basic ammino acids but maybe still a lot of H2O bound to protein (total protein content
in cow milk is approx 3.2g/100ml in every shop in my country, sheep milk is higher in protein and even more deer)
10g of lactose from 100ml of milk seems to be higher than usually also
milks of various mammals (human, horse, cow, deer, goat, sheep, rat) tuky=fats, bielkoviny=proteins, laktoza=lactose
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
I'm from india.Here they use some kind of genetically transformed cows which produces more protein and lactose in milk. Also
Probably I will be having a lot of moisture in both the products. And yes I sundried them probably that's the reason for overweight
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|