Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Sulfuric acid from Ozone generator and Sulfur.
(Not sure if this belongs in beginnings, also there is a simmilar thread there: http://www.sciencemadness.org/talk/viewthread.php?tid=68457)
There are a variety of methods to make sulfuric acid for the home chemist but many of them require comlicated equipment, are expensive or are
prohibitivly dangerous for the less experienced amateur chemist.
One method which seems worth an attempt is the oxidation of sulfur (0) to sulfur (IV) by ozone.
Wikipedia:
"Sulfuric acid can be produced from ozone, water and either elemental sulfur or sulfur dioxide:
S + H2O + O3 → H2SO4
3 SO2 + 3 H2O + O3 → 3 H2SO4"
Ozone generators fed with air, the cheapest option, also produce nitrogen oxides which react vigorously with sulfur too.
https://www.youtube.com/watch?v=demNFwjH-us
Im thinking you would bubble ozone through a suspension of sulfur in water with a fan or aquarium bubbler that doubles as cooling for the generator.
Sulfur is readily available and Ozone generators can be built or bought rather cheaply from ebay
http://www.ebay.com/sch/i.html?_odkw=tube+ozone+generator&am...
These generators supposedly produce 7.5g O3 an hour at optimum conditions. If you let it run for 24 hours and reacted that Ozone with
Sulfur with 100% effeciency it would yield a whoping 377,6g of theoretical Sulfuric acid.
7.5g/h * 24h = 184.4g
S + O3 + H2O = H2SO4
Now, the ozone generator forms nitrogen oxides as a byproduct of the ozone production, but this may be beneficial. The nitrogen oxides could catalyze
the oxidation of sulfur/sulfur dioxide to sulfur trioxide like in the lead chamber process, decreasing the need for ozone and improving reaction speed
and electrical effieciency.
Is this feasible, has anyone tried it?
P.S: This was written very late at night, i will try to fix inevitable errors tomorrow.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
"Optimum conditions" for an ozone generator's rating typically means that the gas coming in is pure O2. Additionally, NOx species can be totally
avoided by using dry air, and probably would not be beneficial since they catalyze the decomposition of ozone as well. Also, I think ozone reacts with
sulfur dioxide to produce SO3 and O2, I'm not sure if O3 can react directly with elemental sulfur or not. Maybe at higher temperatures?
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  | | "Optimum conditions" for an ozone generator's rating typically means that the gas coming in is pure O2. Additionally, NOx species can be totally
avoided by using dry air, and probably would not be beneficial since they catalyze the decomposition of ozone as well. Also, I think ozone reacts with
sulfur dioxide to produce SO3 and O2, I'm not sure if O3 can react directly with elemental sulfur or not. Maybe at higher temperatures?
|
Im pretty sure it oxidizes Sulfur at room temprature. It oxidizes sulfides at room temprature according to multiple sources and even oxidizes silver
at room temprature. I have no doubts it oxidizes Sulfur but i guess ill have to try it.
According to atomistry...
http://oxygen.atomistry.com/physical_properties_of_ozone.htm...
...Ozone is decomposed very rapidly by water. Maybe it would have enough time to react with the Sulfur, otherwise you would have to do it dry, this
might produce Sulfur Trioxide if the humidity is low enough. While it would work it would kind of defeat the purpose of this method.
As for the Nitrogen Oxides, they might decompose the Ozone, especially if they accumulate in the Sulfur/water mix but i dont think it wouald be that
bad. It is not laike you would store the Ozone for a long time, it might take a few seconds to reach the water/Sulfur mix and even then it would still
oxidize athe Sulfur.
Im imagining something like the picture below. The orange thing is supposed to be a fan, the fan is supposed to sit in a soda bottle with the bottom
taken off it blowing air past the blue Ozone generator into a tube. The tube leads to a bubbler or if doing it dry; Sulfur powder. The fan might not
make a higah enough pressure for the bubbler to work but i happen to have a microwave fan laying around. Hopefully my drawing is not too terrible.
Im going to order one of those thaings and try it, if it does not work i will still have an Ozone generator to play with.
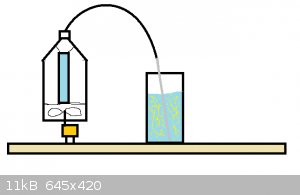
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Have you ever done an ozone reaction before? I have. I was using ozonolysis to oxidize styrene into benzaldehyde. I started with ten grams of
styrene, but eventually had to shut down the reaction, because it was going to take WEEKS. If you don't have pure O2, your ozone generator will
output only a tiny fraction of what it's rated at. And if you do have pure O2, you'd be much better off oxidizing sulfur via the contact process.
There are SO many easier, faster ways of getting sulfuric acid than what you're proposing, which I wouldn't expect to work anyway. The gas flow rate
is really high, and if you're getting SO3 vapors, they'd all be blown away by the huge volumes of air that are constantly circulating through your
apparatus.
Ozone generators work really well for one thing though: getting rid of smells, especially organic ones. Ozonolysis is also pretty neat, but it's too
slow to be practical, unless you have a source of fairly pure O2.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
My quick research appears to verify that the electrolysis of CuSO4 or MgSO4 with inert electrodes (like carbon) produces H2SO4, at least per one
source. I expect it is likely also formed with other hydrogen, sulfur and oxygen acid members of the family as well.
Source: See for example: http://www.instructables.com/id/Make-Sulfuric-Acid-by-Copper...
I would avoid stirring the solution.
I may get a chance to verify this route which would make sulfuric or related acid readily available to many.
[Edited on 17-5-2017 by AJKOER]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
A safe path more in accord with your current setup would be to pass just air/O2 (no O3) into a suspension of finely crushed Fool's gold, FeS2. To
quote a source:
"The oxidation of iron sulfide pyrite by molecular oxygen produces iron(II), or Fe2+:
FeS2 + 7/2 O2 + H2O → Fe2+ + 2 SO42- + 2 H+
The Fe2+ can be further oxidized to Fe3+, according to:
Fe2+ + 1/4 O2 + H+ → Fe3+ + 1/2 H2O
and the Fe3+ so produced can be precipitated as the hydroxide or hydrous oxide. The equation for the formation of the hydroxide is:
Fe3+ + 3 H2O → Fe(OH)3 + 3 H+ "
Link: https://www.cs.mcgill.ca/~rwest/wikispeedia/wpcd/wp/s/Sulfur...
A more accurate rendition revealing the complex system of possible underlying reactions, in my opinion, in light of the lack of consensus on the
reaction mechanism (involving, I would guess, electrochemical, radicals, and surface chemistry), please see also: https://books.google.com/books?id=Klc5GN2uDwoC&pg=PA33&a...
Given the photochemistry surrounding FeS2 (used in solar panels), adding sunlight (promoting superoxide radical creation from Fe(lll) + hv = Fe(ll),
Fe(ll) + O2 = Fe(lll) + .O2-, and at low pH, Hydrogen peroxide, from 2 H+ + .O2- = H2O2, for related syn-FeS2 chemistry, see http://pubs.acs.org/doi/abs/10.1021/acsami.5b09919), a small amount of sea salt (an electrolyte also increasing ionic stength), solution
shaking/agitation (to promote dissolved oxygen formation) and some acidic Copper(ll) chloride (creating a redox couple based equilibrium with Fe(ll)
leading independently (from an oxygen path) to Fe(lll) and also Cu(l), and the reverse reaction, Fe(ll) and Cu(ll) ), I would guess could increase the
reaction rate leading to the creation of H2SO4 with transition metal impurities.
[Edited on 20-5-2017 by AJKOER]
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I'm really interested in this as I have a 5L/min O2 concentrator ATM. I know I can build a generator and probably have everything I need but I'm lost
on which kind to look at. There are a few different styles, I think 3 main types. Some use a plasma arc - similar in size to a spark plug but a
little larger. Another type is using a glass tube, wire around the outside and foil (or similar) inside the tube and pass air through this. I think
the glass is the weak point and quartz is recommended. The last type is using glass plates and foil on them.
I've also heard that laser printers/copiers generate O3 and I think this is done with the electric wire that is used to heat the toner to fix it to
the paper.
If anyone has interest in building a high output O3 generator (more than 5-10g/hr) I'm ready to start and have the parts to start. HV transformers
(2Kv - 25Kv), flyback transformers from all kinds of TV's and other electronics, gas furnace transformer (6-8Kv - used for spark plugs I think??), HV
diodes & caps, tons of transistors, etc. I should be able to build something if anyone has a good idea of which type is best suited for use with
concentrated O2.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Honestly if I see anyone posting about using ozone while being afraid of NOx... let's just say a certain alarm goes off in my head.
Ozone is NOT less dangerous than NO2. It's a blue 4 on the NFPA diamond and it will kill a human in 30 minutes at 50 ppm. If you can't contain NO2 you
can't contain ozone... period. NO2 is actually a better choice than ozone when possible IMO since it can be condensed.
The reason ozonolysis takes forever with consumer-level O3 generators isn't thermodynamic limitations. The O3 output of the machine is limited so it
won't kill you. That's the key.
[Edited on 19-5-2017 by clearly_not_atara]
|
|
|
tshirtdr1
Harmless

Posts: 31
Registered: 12-12-2014
Member Is Offline
Mood: No Mood
|
|
Tesla Coil
I tried doing an ozonolysis with an ebay ozone generator, and it didn't work. I wouldn't waste my money. I think what you want is a Tesla coil with an
O2 source feeding oxygen into it. I still don't know if your idea will work, though. Just saying that the ozone generators are not powerful enough to
do much good, as others have stated.
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
Quote: Originally posted by clearly_not_atara  | Honestly if I see anyone posting about using ozone while being afraid of NOx... let's just say a certain alarm goes off in my head.
Ozone is NOT less dangerous than NO2. It's a blue 4 on the NFPA diamond and it will kill a human in 30 minutes at 50 ppm. If you can't contain NO2 you
can't contain ozone... period. NO2 is actually a better choice than ozone when possible IMO since it can be condensed.
The reason ozonolysis takes forever with consumer-level O3 generators isn't thermodynamic limitations. The O3 output of the machine is limited so it
won't kill you. That's the key.
[Edited on 19-5-2017 by clearly_not_atara] |
To support this point, note that ozone and sulfur dioxide are very chemically similar, because S and O are in the same group--and SO2 is toxic as many
people know, yet O3 is even more toxic because of its stronger oxidizing power
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by clearly_not_atara  | | The reason ozonolysis takes forever with consumer-level O3 generators isn't thermodynamic limitations. The O3 output of the machine is limited so it
won't kill you. That's the key. |
No... it has a lot more to do with the fact that when you use air as your input, O3 levels are far lower than when using pure oxygen, which is what
you need to use if you're going to be needing significant amounts of it. There are many different levels of O3 generators available to anyone who
wants to buy them, and most are rated at the O3 production rate with pure dry O2 as an input. People get them assuming that's the O3 production rate
for air as an input and then get surprised when it takes forever.
Lots of things can be purchased that could kill someone when misused. That's what warning labels and manuals are for.
| Quote: | | To support this point, note that ozone and sulfur dioxide are very chemically similar, because S and O are in the same group--and SO2 is toxic as many
people know, yet O3 is even more toxic because of its stronger oxidizing power |
You're totally wrong here. What's the oxidation state of the atoms in SO2? Ok, now what are the oxidation states of the atoms in O3?
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
https://www.arb.ca.gov/research/indoor/ozone.htm
https://www.jenesco.com/EPA-Regulations.html
| Quote: | | The Food and Drug Administration (FDA) requires ozone output of indoor medical devices to be no more than 0.05 ppm (parts per million).
|
It's limited by government regulations so that it won't kill you. IDLH for ozone is 5 ppm. For chlorine it's 10 ppm, and for NO2 it's 20 ppm.
Ozone is roughly four times as dangerous as nitrogen dioxide. You can certainly exceed 5 ppm starting from air -- it's just stupid to do so. Notice I
said consumer-level ozone generators.
https://en.wikipedia.org/wiki/Ozone#Production
| Quote: | | The regime of applied concentrations ranges from 1% to 5% (in air) and from 6% to 14% (in oxygen) for older generation methods.
|
5% = 50000 ppm. The FDA maximum ozone output of a consumer ozone generator is a million times lower than the maximum achievable by
ionising air.
[Edited on 3-8-2017 by clearly_not_atara]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
And your quote specifies indoor medical devices. None of the ozone generators I've ever purchased has been a medical device. They do have
warnings on them to only use in unoccupied spaces though. Attics, basements, etc. They mostly seem like they're meant to be used for reducing
allergens and strong odors in preparation for cleaning a room where those things are a problem.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
Quote: Originally posted by Melgar  |
You're totally wrong here. What's the oxidation state of the atoms in SO2? Ok, now what are the oxidation states of the atoms in O3?
|
Yes I know they're not chemically exactly analogous (O3 has oxidation state 1.5 in each oxygen bond vs SO2 has 2), I just meant as a kind of
"reference point" or memory device for those who might not really recognize the toxicity of ozone, as clearly_not_atara said--because "it's like
sulfur dioxide but even worse" or something.
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
And to make a post that's a bit more on-topic for the thread, could NO and NO2, which are also generated from atmospheric electrical discharge, be
useful in sulfuric acid production? Perhaps they, being powerful oxidizers like ozone, could oxidize SO2 to SO3? And if the reaction between the gases
isnt fast enough at normal conditions, maybe heating the gases could get it to react. Otherwise, I wonder what would happen if you sent the gas
combination through another electrical discharge.
btw I intend to try some of these experiments in the future (if they're not too dangerous) and I'll post the results.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by physics inclination  | And to make a post that's a bit more on-topic for the thread, could NO and NO2, which are also generated from atmospheric electrical discharge, be
useful in sulfuric acid production? Perhaps they, being powerful oxidizers like ozone, could oxidize SO2 to SO3? And if the reaction between the gases
isnt fast enough at normal conditions, maybe heating the gases could get it to react. Otherwise, I wonder what would happen if you sent the gas
combination through another electrical discharge.
btw I intend to try some of these experiments in the future (if they're not too dangerous) and I'll post the results. |
That's basically the lead chamber process in a nutshell. The NOx is recycled as well, so not much of it is needed.
The point I was trying to make with ozone though, is that it's a semi-stable free radical, whereas SO2 is not. Free radical chemistry has a totally
different set of rules compared to the chemistry you're probably most familiar with.
Also, the oxidation state for all atoms in O3 is 0, because they're all the same atom, so it was kind of a trick question.
[Edited on 8/3/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Not hard to make a high potency generator with some stainels steel, auto ignition coil, quartze tubing, 55 driver and a crap load of silica gell and a
co2 absorbent.
Clean dry air is important, removal of co2 just helps a bit more for silent arc discharge style
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
I found this article, which says you can indeed directly oxidize SO2 to SO3 via a non-thermal plasma discharge through the SO2 gas mixed with air:
"An alternative method is the oxidation of SO2 to SO3 in the gas phase. SO3 can be removed easily in water solution [this produces sulfuric
acid/oleum]. One of the technologies to oxidize sulfur dioxide is the treatment of polluted gases with non-thermal plasmas. Active particles in
non-thermal plasmas such as atomic oxygen will oxidize SO2 molecules.
In non-thermal plasmas the energy (temperature) of electrons is much higher than that of the heavy particles (atoms, molecules, ions). As a rule the
energy of electrons in non-thermal plasmas is of the order of 1 eV (corresponding to 11 600 K), while the molecules and ions remain more or less
at ambient temperature. Most important sources of non-thermal plasmas are (pulsed) corona and dielectric barrier discharges (DBDs) as well as electron
beams with much higher electron energies. All these sources are able to operate at ambient pressure."
So you don't even need to make NOx in the first place, using these methods.
here's the sci-hub copy to download the full article, I didnt directly put it in this post because maybe it would be too large file idk:
https://sci-hub.ac/10.1088/0963-0252/16/3/005
[Edited on 8-4-2017 by physics inclination]
|
|
|