MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Spinning DCM Droplet
I saw this interesting article today - https://www.livescience.com/61417-spinning-dichloromethane-c...
From the article:
| Quote: | The paper's thesis was simple: when you release a droplet of dichloromethane (DCM) solvent into a beaker of soapy water, it looks really, really cool.
...
To set up the experiment, Steinbock and his fellow researchers filled several beakers with various concentrations of water and a common lab
disinfectant called CTAB. Using a pipette, they added a single drop of DCM — a colorless liquid sometimes used as a degreaser — to each beaker,
and filmed the results. Each trial took about 20-30 seconds total, and was visible with the naked eye.
Each drop of DCM, which has a relatively low boiling point, began evaporating as soon as it left the pipette. But the surprises began when the
droplets touched the soapy water solution.
"DCM has a higher density than water, so you'd expect it to sink right away," Steinbock told Live Science. "But instead, as soon as it touches the
water, part of it spreads out and creates this sort of film that holds the droplet on the surface of the water… it’s like a boat that holds the
droplet afloat.”
...
Despite this boat-like film, a small part of the droplet does begin to sink. It's not visible from the top-down vantage point of this GIF; however, a
tiny jet of falling bubbles forms underneath the droplet once it touches the water. The falling DCM jet slowly shrinks the droplet's volume, but also
causes it to spin. "It’s a little bit like when you flush a toilet," Steinbock said. "The water has a tendency to start to rotate and twist. And
that triggers the rotation of the drop that we begin to see."
Within a few seconds, the droplet is at once floating, rotating and evaporating. As a result of these combined forces, smaller droplets eventually
start disengaging from the edge of the larger droplet. But instead of sinking themselves, they shoot out radially, moving straight ahead over the
surface of the film until they, themselves, evaporate.
"These droplets are self-propelled," Steinbock said. This is due to a phenomenon called the Marangoni effect, which states that a liquid with a high
surface tension will pull more strongly than a liquid with a low surface tension. This difference in tension creates a force on the system that can
lead to motion.
As the DCM in the experiment begins to evaporate, the droplet's surface tension lowers from the outside in. Smaller droplets begin to form at the
large droplet's edge, until the relatively high surface tension of the surrounding water pulls the small droplets away in what Steinbock calls a
"ballistic" trajectory. Each individual droplet moves straight ahead until its surface tension becomes equally unstable, leading to further
fragmentation. Eventually, the droplets split so many times they can no longer be seen. |
The gif is extremely cool. Seems very simple, and I'd like to give it a try when I have time.
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I like it. What temperature is the water?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
There aren't any references to the actual paper unfortunately. I'd think hot water, to facilitate evaporation?
It's crazy that the DCM is floating on the water, especially after adding soap which should decrease surface tension!
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
I doubt it would be too hot... DCM boils around 39 to 40 C Hot water might boil it off too quick - it said 'evaporates' rather than boils off. It
evaporates pretty quick at room temp iirc.
DCM - is that the one that smells lovely, or is that ethyl ether? One of them smells really nice anyway, or both. lol. It has been ages since I used
either. I remember from my uni days the amazing smell in the corridor when people were rotovapping DCM or Ether. :-) (might have been diethyl ether,
not sure now)
[Edited on 16-1-2018 by DrP]
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Found the note, it has all the details.
There is a range of forms of the drops:
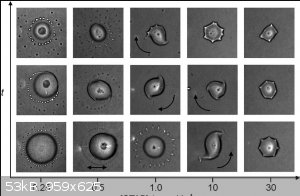
from DOI: 10.1002/anie.201104261
Attachment: ldrops.pdf (851kB)
This file has been downloaded 215 times
They used cetyltrimethylammonium bromide surfactant. Perhaps it will work with others. I only have Triton or washing up liquid.
[Edited on 16-1-2018 by wg48]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
This is great; I was hoping someone would find the original paper. Thanks wg48!
From the Experimental section (emphasis mine):
| Quote: | All chemicals used are of analytical grade. Cetyltrimethylammonium bromide (CTAB; Aldrich, �99%) and dichloromethane (Aldrich, HPLC grade) were used
as purchased. The water is ultra-pure (resistivity >17 MWcm). All experiments are carried out at room temperature. The CTAB
solution (25 mL) is filled into a cylindrical container (diameter 70 mm) and a single 25 mL drop of CH2Cl2 is carefully placed onto the solution
surface using a pipette. The system is then covered by a glass plate to reduce matter exchange between the gas layer above the fluid and the
surroundings. It is illuminated with white light and the shadow of the floating, lens-shaped drop is monitored from the top with a video camera. Note
that the densities of CH2Cl2 (1.33 gmL�1) and water-saturated CH2Cl2 are larger than the density of water.[13] The solubility of dichloromethane in
water is 13 gL�1, which corresponds to a volume 250 mL in 25 mL.
The reproducibility of the experiment is greatly increased by saturating the organic liquid dichloromethane with water.
|
Sounds pretty reproducible, except for my lack of CTAB. Since it's just a surfactant, maybe regular liquid dish soap would work.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I have now tried it using washing liquid up or Magnum rinse aid at a range of concentrations from zero to undiluted. With the washing up liquid at all
concentrates tested the drops fell through surface film. With weak solutions of the rinse aid again most drops fell through the surface film
immediately with a few staying upto a second.
I tried again with fresh solutions and dichloromethane shaken with water. I also switched to a finer pipette. Undiluted rinse aid supported most of
drops but no activity.
With the rinse aid diluted 4:1, no obvious motion of the drops but just visible surface waves surrounding drop. Then I discovered if the drops are
dropped on to the surface from about 10mm some of the drops form a perfect small sphere (about 2mm diameter) that then dart about the surface very
rapidly for up to several seconds before abruptly sinking in to the liquid. No obvious rotation but it would be difficult to see any.
More experiments are needed and in particular a good method of visualising drop with magnification. I was using the reflection from a desk lamp.
Colouring dichloromethane may be effective.
I could not find the ingredients of the Magnum rinse aid. Below is the ingredients from Finish rinse aid that may be similar.

[Edited on 18-1-2018 by wg48]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Any one interested in buying Cetyltrimethylammonium chloride? Say £10/500ml + shipping from the UK. Yes its not the bromide but I could only find
the chloride at a reasonable price and in a minimum of 1l. It works as a phase transfer catalyst as well as powering the spin of dichloromethane
drops.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Just saw your experiments. Disappointing that it didn't seem to work with a regular surfactant. I'd love to try it with the real thing (or close to
it), but I certainly don't need 500mL of it. I'd use a few mL for the experiment, then have 495mL I wouldn't know what to do with!
I had no luck finding the bromide either. Strange, since they said CTAB is a "common lab disinfectant."
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Interesting.
The original video looks like it is a micellar effect going ape due to the low BP of the DCM.
deltaH suggested a thing when i asked, and it appears his old Uni excelled at making nanometre scale catalysts using exactly this effect.
Edit:
The interesting bit is the micelle formation, not the spinning blob.
[Edited on 22-1-2018 by aga]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
So I have tried a set up with web cam with the lens screwed well out to focus at about 80mm. Its difficult with the cam tie wrapped to a desk lamp
looking down into a mug of water. No attempt at spinning drops just a test of the set up video. I had to sprinkle some photocopy powder on the water
surface to get the focus right. So here are two snaps from the video and the video. Its not exciting but it suggests more magnification is needed.
 
Attachment: testsetup.wmv (7.2MB)
This file has been downloaded 529 times
Sorry the clip does not include the sprinkling of the green dye
|
|
|