woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Rotational barrier between cis and trans rotamers
While reading about nitrite esters I found some intriguing information about methyl nitrite (Wikipedia also mentions it briefly). The compound can
exist in two different rotational "isomers". They are no true structural isomers, nor are they stereo-isomers.
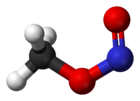 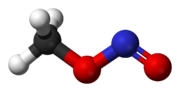
The structures are the same, except that -N=O part of the -O-N=O group is rotated around the O-N bond by 180 degrees.
Can someone explain in easy to understand terms why there are two rotational isomers (so-called rotamers)? Why isn't there free rotation around the
O-N bond? As there are only two rotamers, I assume the C-O bond does have free rotation. The main difference between these two rotamers then is that
in the cis-form (the left one) the =O is closest to the CH3-group and in the trans-form (the right one) it is furthest away (rotation around the C-O
bond does not change the distance between the =O and the CH3 group).
The energy barrier between these rotational positions is 45 kJ per mole. This is so much that under normal conditions the one form does not easily
change into the other form.
For me, this has no practical meaning, it is just curiousity why I ask this. I made methyl nitrite quite a few times, just as a cute curiousity, which
burns with a grey flame when ignited and I am quite sure that in practical experiments I will never notice the diference between these two rotamers.
It just is a colorless gas to me, regardless of which rotamer I have in front of me.
|
|
|
j_sum1
Administrator
       
Posts: 6220
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
At a guess, I would put it down to interaction between the unpaired electron clouds on the O and the N. I would expect that due to repulsion the trans
rotameter would be the lower energy state. But I can visualise the electron cloud on the N sandwiched between the two clouds on the O restricting
free rotation from the cis to that lower energy position.
This is just a guess mind you.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It's because of the existence of a minor resonance structure, where the double bond is between the nitrogen and the other oxygen, with a positive
charge on one oxygen and a negative on the other. Because of this resonance structure, the O-N bond doesn't exhibit perfect single bond character, and
is actually somewhat rigid
|
|
|
j_sum1
Administrator
       
Posts: 6220
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
That makes even better sense. Thanks for that, Texium.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by Texium (zts16)  | | It's because of the existence of a minor resonance structure, where the double bond is between the nitrogen and the other oxygen, with a positive
charge on one oxygen and a negative on the other. Because of this resonance structure, the O-N bond doesn't exhibit perfect single bond character, and
is actually somewhat rigid |
This is the best explanation I have read about this phenomenon 
Many web pages write about this, but none of them makes it really clear. They talk about ab-initio calculations, orbital mechanics and other fancy
stuff, but your explanation really ticks.
|
|
|