Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
NaDCC/TCCA reaction with ammonia
I have always had a fascination with NaDCC and TCCA . So much nitrogen and anger in a pair of compounds so easily obtained so why not add more? The
most obvious way to do that is to try reacting it with ammonia to replace the chlorine with amine groups. Not only is this interesting from an
energetics perspective but also as a potential route to hydrazine.
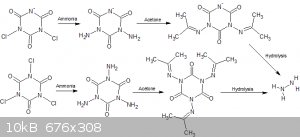
I tried the reaction of ammonia and NaDCC;
NaC3O3N3Cl2 + NH3 = NaC3O3N3(NH2)2 +
NH3*HCl
NaC3O3N3(NH2)2 + CO(CH3)2 =
NaC3O3N3(NC(CH3)2)2 + H2O
With an eight times excess of ammonia. The procedure was:
100g of solution of which 0.245 was ammonia was added to a beaker, covered with plastic wrap, and chilled in a freezer. 10g of NaDCC was dissolved in
100ml distilled water and frozen to a slush in the same freezer. The slushed NaDCC solution was added dropwise to the ammonia with vigorous stirring
with effervescense as a white precipitate formed. After the addition was complete the solution was again chilled and then vacuum filtered. 20g of
acetone was added to the filtrate and it was again placed in the freezer where it remains now. It looks as if it is willing to precipitate something.
The precipitate seems fairly insoluble in water, is soluble in both 30% Hydrochloric acid and 60% acetic acid, does not seem to react with a KMnO4
solution nor with Cupric Sulfate, even when dissolved in acid. When heated it first releases what looks like water and seems to melt at somewhat below
dull red heat where it begins to bubble and melt turning into a slightly brown liquid. Presumably Sodium Carbonate. I am not sure what it is but i
doubt it is my desired product. Supposedly Ammonium Cyanurate is soluble so i doubt that is it either. I would not be suprised if the target compound
is insoluble tough from extensive hydrogen bonding such as in Melamine Cyanurate or it might be some crap formed by self condensation.
I chose the NaDCC reaction because of the solubility of NaDCC and because my TCCA contains both boric acid and Copper Sulfate which you do not want in
proximity to anything with a N-N bond. Woelen described the reaction as vigorous which i would agree with but it did not seem to produce much heat, i
managed to add all the NaDCC without it becoming warm to the touch. It may be much more vigorous if you no not use excess ammonia and let Nitrogen
Trichloride form though.
http://www.sciencemadness.org/talk/viewthread.php?tid=24088&...
Besides ammonia urea and guanidine could be interesting to get some very nitrogen rich compounds.
[Edited on 15-9-2018 by Σldritch]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I am pretty sure you will end up with nitrogen trichloride as a product.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Your initial solid product is cyanuric acid. A little digging on Google would have told you that. The equations you have written are completely
non-sensical because things just do not work that way.
AvB
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AvBaeyer  | Your initial solid product is cyanuric acid. A little digging on Google would have told you that. The equations you have written are completely
non-sensical because things just do not work that way.
AvB |
Tried dissolving TCCA in excess HCl and it does not act the same, i was confused by google giving me results for KCNO which obviously hydrolyses much
quicker but reality beats google.
And no it will not produce any significant amounts of Nitrogen Trichloride because the pH is too high and the ammonia is in excess.
Apparently higher temperatures favor the reaction of Chloramine and ammonia to form hydrazine, maybe i would get product if i increased the
temperature.
[Edited on 15-9-2018 by Σldritch]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
In my experiments I added solid Na-DCCA or solid TCCA to ammonia (12%). This reaction is VERY violent. If you add solutions of the compounds to each
other and you do this slowly with ice cold solutions and stirring, then I believe that the reaction is manageable quite well. I never thought about
the reaction products in that reaction though. I can imagine formation of cyanuric acid (or a sodium salt of this) and hydrazine. If the reaction mix
is allowed to heat up, then I expect just nitrogen and cyanuric acid/sodium cyanurate.
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I am starting to think it is a sodium salt too but i have heard many seemingly condradicting solubilities for Sodium Cyanurates, probably because
there are several. And to make it more confusing Hydrazine Cyanurate is supposedly insoluble though i can not find the paper now. Also the filtrate
fizzed which may indicate the precence of chloramine and/or hydrazine. It seems like a complex mixture of various cyanuric acid derivatives and salts.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
TCCA and water forms HOCl.
The action of NH3 + HOCl = NH2Cl + H2O
Chloramine is nasty and can induce asthmatic conditions in healthy people.
With excess HOCl, the above reaction progresses to NCl3.
|
|
|