crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Nitration of xylene
So I am interested in the nitration of xylene to from xylene to MPX to DPX to TPX sometimes called TNX. Im not quite sure whether xylene like you buy
at the hardware store is o-Xylene m-Xylene or p-Xylene. Apparently p-Xylene would be the best because it has only one mononitro and trinitro isomer.
I have some information but not enough to have the whole synth. This would have to be carried out in a three step process. Here is what I have:
1. The mononitration of p-xylene can be easily carried out at 30°C. Nitro-p-xylene is easily nitrated to dinitro-p-xylene at a temperature of 80°C.
The trinitro-p-xylene can be obtained at 120°C
2. For the nitration of nitro-p-xylene, it would be expected that the mixed acid would be less concentrated than for the
dinitration of toluene but more concentrated than for the mononitration of p-xylene. Likewise, the temperature of nitration should be lower than for
dinitration of toluene but higher than that used for the mononitration of p-xylene.
So what do you think? Ideas? synths? Have any of you successfully done this?
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Hardware store xylene is a mixture of ortho, meta, and para xylenes. I have alternative heard that it is more a fraction of hydrocarbons than any
specific compound it's just that the fraction has a high percent of xylene in it. This is refuted by some MSDS' though. I have heard from some
sources that pure xylenes of the ortho/meta/para variatey spontaneously inter-convert giving you a mixture at the end of the road. However since you
can buy specific isomers if there is any interconversion it must be slow unlike some of the pure hexane isomers that contain a warning on the label
stating that it will degrade over time.
|
|
|
tumadre
Hazard to Others
  
Posts: 171
Registered: 10-5-2005
Member Is Offline
Mood: No Mood
|
|
I just looked at the gallon of xylene in front of me, it says: "upper limit 20% ethyl benzene".
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That means up to 20% toluene! It is obviously a "technical" or "industrial" grade, not reagent grade, containing a fraction of the three mixed
xylenes plus some toluene, from fractionation of aromatics in oil refining. It may also contain a small amount of benzene, and possibly of durene
(1,2,4,5-tetramethylbenzene), pentamethylbenzene, and hexamethylbenzene. The last of these cannot undergo aromatic nitration at all, and for the other
two (other than benzene) it would be difficult due to steric factors
Edit: Oops, I misread "ethylbenzene" as "methylbenzene" (toluene). The three xylenes happen to be isomeric with ethylbenzene, and very similar in
boiling points. This also opens up the possibility of small amounts of other ethyl-substituted benzenes (diethyl and ethylmethyl etc), along with
toluene.
[Edited on 3-3-08 by JohnWW]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Toluene is methylbenzene. Ethylbenzene could be regarded as an isomer of xylene.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
Xylenes are specified by boiling range and as such are available in several "purities".
The "BTX" (benzene, toluene, xylene) fraction of crude oil
is specified in the same way. Several grades of benzene
are also available by boiling range as is toluene.
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
OK I finally got around to trying this.
First 20mls 98% sulfuric acid was chilled and slowly mixed with 16g potassium nitrate to this 10ml xylene was slowly added a mash formed with little
blobs of red oil the mixture was slowly heated to about 30-35C where it formed an orange red mash which was stirred every so often. After about 1-1/2
hour’s 500ml boiling water was poured on the mash and the water turned pale yellow and a oil was noticed on the bottom. The top layer was decanted
and replaced with fresh water twice and the mix was allowed to cool and settle for about 1 hour. Then about 11mls opaque yellow oil was extracted with
a syringe this settled and 1ml water was separated and discarded.
The 10mls oil was added to 30mls sulfuric acid mixed with 10mls nitric acid this was heated to about 60-70C and then dumped into 500ml cold water. An
insoluble mass of crystals floated to the top this was washed with water then dried. The crystals are pale yellow in color.
Here is my problem next I added the yellow crystals to another portion of 30mls sulfuric acid mixed with 10mls nitric acid this was heated to 100-110C
then crashed with 500ml water. An insoluble mass of crystals floated to the top this was washed with water then dried. The crystals are pale yellow in
color.
These crystals appear identical to the previous ones. They melt at above 100C into a clear yellow liquid they deflagrate with a low sooty flame and
leave carbon residue.
My question is was the second nitration strong enough to turn the mononitroxylene into trinitroxylene? OR was the third nitration too weak to turn the
dinitroxylene into trinitroxylene?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Look at what you are nitrating when you use solvent grade xylene. The ethyl benzene resembles toluene, save that it gives a slightly higher
proportion of the para isomer in the mononitration.
The xylenes start showing some steric crowding after dinitration. Orto-xylene the least, but that third nitro group will always be going in next to
another nitro, or between two other substitutes.
[Edited on 20-3-2008 by not_important]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Missing post of xylene of two weeks ago writing by my. Sitting around for about 5 hours in a Interetkaffe and writing my finger dead.
I`m so much of       . .
Post deleted my unknown. 
50 lines and more, next mounth maybe more ! To costly !
Every love is a little inspiration - every day is
a new live !
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Well take the hint Mason and dont come back.
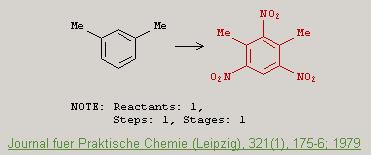
Heres an article that supposedly gives the preparation of m-TNX via one step nitration.
Karl Egil Malterud "Improved Synthesis of 4,6-Diamino-2-nitro-m-xylene by Reduction of 2,4,6-Trinitro-m-xylene" <i>Journal für Praktische
Chemie</i>, 321(1), 175-176 (1979)
http://dx.doi.org/10.1002/prac.19793210127
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
I know this thread is old but I have some new info.
After rereading my source and COPAE I have come up with an idea according to COPAE:
In m-xylene the two methyl groups agree in activating the
same positions, and this is the only one of the three isomeric
xylenes which can be nitrated satisfactorily to yield a trinitro
derivative. Since the three isomers occur in the same fraction of
coal tar and cannot readily be separated by distillation, it is
necessary to separate them by chemical means. When the mixed
xylenes are treated with about their own weight of 93 per cent
sulfuric acid for 5 hours at 50°, the o-xylene (b.p. 144°) and the
m-xylene (b.p. 138.8°) are converted into water-soluble sulfonic
acids, while the p-xylene (b.p. 138.5°) is unaffected.
My source says p-xylene not m-xylene is the best isomer to nitrate because it only has one mononitro and trinitro isomer. Even if this wasn't true I
don't have an autoclave to convert the sulfonic acid back to xylene.
So I plan to take store bought xylene treat it with sulfuric acid at 50C for several hours separate the aqueous layer and be left with pure p-xylene
then I can nitrate it to mono dinitro or trinitro quite easily.
Axt I cant read the document.
Also I took my old nitrated xylenes as I posted earlier and washed them with acetone to get an almost perfectly white fluffy powder with tiny long
needle like crystals.
[Edited on 20-10-2008 by crazyboy]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by crazyboy
These crystals . . deflegrate with a low sooty flame and leave carbon residue.
was the second nitration strong enough to turn the mononitroxylene into trinitroxylene ? |
Reaction products are smokey but not sooty, so that alone means you likely have
a di-nitro product if it does not detonate from a sharp blow. Deflegration does not
reveal anything about the constituents of your product.
Aryl, Arene, Aromatic chemistry is bewildering to all but experts
This might help you, in particular scroll down to middle of page.
http://www.cem.msu.edu/~reusch/VirtualText/benzrx1.htm#benz1
See Urbanski vol I , chap X , pg 395 ,
NITRO DERIVATIVES OF HIGHER BENZENE HOMOLOGUES
.
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Thorpe's method
I remember nitrating Home Depot-bought xylene quite a number of years ago. In fact, I probably still have some of it around. I followed the method
prescribed by Thorpe in the seventh volume of his Dictionary of Applied Chemistry (page 523 of the 1930 edition.) It is a two step process (I
only followed the first part, which is the one he gives details for.) The proportions are:
50 grams of commercial xylene (mixture of three isomerides)
500 grams of mixed acid (2 parts of nitric acid, D 1.52, to 3 parts of sulphuric acid, D 1.84.)
With a dropping funnel I gradually added the xylene to the room-temperature acid mix while stirring. The final products I got after adding water,
separating and washing, were a white solid substance, mostly in long needle-like formations, which is the impure TNX, and a smaller amount of a
yellowish-orange oily substance (liquid nitroxylene, according to Thorpe's description.)
According to Thorpe, this first nitration yields a product that contains small amounts of dinitroxylenes and therefore is "useless as an explosive"
(by itself, I suppose, because Davis seems to say that such mixtures of nitrated xylenes can be used in dynamites, and with ammonium nitrate for
explosive shells.) Thorpe then says that a second nitration (unfortunately he does not say in what proportions) yields white crystals capable of
detonation by 0.15 - 0.2 grams of silver azide.
[Edited on 21-10-2008 by Blasty]
|
|
|