| Pages:
1
2
3
4
5 |
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
That's good news although you can use thrimethylglycine instead of choline chloride which is a supplement and easy to find.
You are right about this method I found it here:
http://www.pharmacopeia.cn/v29240/usp29nf24s0_m17300.html
The page says you can dry it at 120 C for 2 hours.
[Edited on 2-5-2015 by gatosgr]
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
You can use vacuum to dry salts (can't you, or am I mistaken?)
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
Yes, vacuum should dry salts. I am more concerned about isolating my choline chloride from solution without decomposing it as it is deposited on the
sides of the vessel. I may try putting it under vacuum and seeing if the water boils off as I have a 25 micron pump.
Alternatively, does putting a solvent such as acetone into a saturated solution of choline chloride force it to precipitate out if it is insoluble in
the second solvent? I know that people reclaim choline chloride from a DES with urea using acetone, but I am unsure regarding miscibility mechanics if
anything would happen.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
The pure choline chloride may be recovered from this aqueous solution by distilling off the water in the solution, preferably under reduced pressure.
The choline chloride which is recovered will have a purity of 98% or better and may be converted to the U. S. P. grade of choline chloride merely by
one recrystallization from a solvent such as isopropanol, isobutanol, etc.
You can also use a dessicator bag https://www.youtube.com/watch?v=XJFfS_YbbYI but it will take some time.
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
That sounds like the best option right now. I don't actually have a vacuum adapter for distillation though, as due to some quirks of glassware
obtaining I only have a graham condenser and a 180 degree adapter so that the setup ends pointing down.
I will make sure to update when I finally make a DES, as I have plans for a sodium chloride cell that I think would be most entertaining.
Unfortunately I will not be able to continue with the process for another week as school gets really crazy this time of year 
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
You can use a dessicator bag https://www.youtube.com/watch?v=XJFfS_YbbYI
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
The issue I have with dessicators is the large volume of water that I need removed from the choline chloride solution. I have about 600 mL of water
that I need to remove and that would take a ridiculous amount of dessicant and time to accomplish. If you have done a large scale dessication in the
past I would love to hear how you did it, but currently I have never heard of the process being used in a situation like this.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Perhaps a 5kg bag of broken rice sold as pet food could be dried in a normal oven, and then put inside a bin-bag along with your 600ml of water ?
[Edited on 5-5-2015 by aga]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Wiki (I know, not the best source) lists the decomposition temperature of choline chloride as 302 C. So why can't you just boil away the water? If you
want to be super careful, boil away until crystals start to form, allow to cool and precipitate more crystals, filter off, then dry the damp crystals
the rest of the way in a desiccator.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Well regardless of how ridiculous this sounds you can make a cheap vacuum pump from an injection maybe you can lower the boiling point a bit although
I havent calculated the pressure needed.
https://www.avs.org/AVS/files/10/1043c4c6-597a-498f-a45d-7f1...
thank me later
[Edited on 5-5-2015 by gatosgr]
[Edited on 5-5-2015 by gatosgr]
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
Oh, I have a vacuum pump, I just don't have any of the requisite glassware for a vacuum distillation. This is still very interesting, I'll definitely
save that for future use. Do you know what the maximum pressure achievable is with this pump?
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Measurements indicate that this pump is capab
le of producing a vacuum of less than 5%
of atmospheric pressure (~.8 psi, ~40 torr).
This displacement (~ 60 sccm) and ultimate
pressure (.05 atmosphere) is adequate for
many of the standard vacuum demonstrations
(for example, crushing cans and bottles, expa
nding a marshmallow, etc.). It takes ~12
strokes to collapse
a 500 ml water bottle.
|
|
|
Arun2642
Harmless

Posts: 2
Registered: 2-7-2015
Member Is Offline
Mood: No Mood
|
|
Where did you find that trimethylglycine could be used as an alternative to Choline Chloride, I just tried making a DES with trimethylglycine 1:2 urea
and trimetylglycine 1:1 urea. Both crystallized well above room temperature. The method I used was to melt the urea in an erlyn-myer flask, add the
trimetylglycine, and swirl and reheat until it all formed a homogeneous solution. Is there a different procedure I should follow?
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
I have been able to create a Type IV deep eutectic solvent by heating a 1:1 molar ratio of AlCl3 and Urea under oil (the paper recommends nitrogen,
but I don't have access to any). It worked well as a supercapacitor electrolyte for me, but when it gets down near room temperature it gets quite
viscous. I used this paper as a reference: https://www.sciencemadness.org/whisper/files.php?pid=404131&...
nlegaux
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arun2642  |
Where did you find that trimethylglycine could be used as an alternative to Choline Chloride, I just tried making a DES with trimethylglycine 1:2 urea
and trimetylglycine 1:1 urea. Both crystallized well above room temperature. The method I used was to melt the urea in an erlyn-myer flask, add the
trimetylglycine, and swirl and reheat until it all formed a homogeneous solution. Is there a different procedure I should follow?
|
The procedure I follow is to mix the two powders and heat it in a glass tube stirring with a glass thermometer , I normally don't let it exceed 110
degrees because compounds get pyrolyzed at that temperature.
If you're getting crystals then the ratio isn't right try trimethylglycine with something else.
[Edited on 3-12-2015 by gatosgr]
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
delete this double post
[Edited on 3-12-2015 by gatosgr]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I have boiled down choline chloride solutions in an open pan. Eventually, it gets very viscous and forms films on the surface, this is the end-point,
because on cooling the whole lot crystallised into crystals.
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
I am having trouble understanding what you mean, deltaH. Choline chloride decomposes fairly easily and I feel that this would easily decompose it into
fishy trimethylamine. Also, if it is getting viscous but there is still water, when you ultimately cool it down there will still be water in your
batch of crystals. I suppose you could try vacuum filtration of this to remove as much liquid water as possible from the precipitate...
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Just order a bag of trimethylglycine seriously..
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by DeIonizedPlasma  | | I am having trouble understanding what you mean, deltaH. Choline chloride decomposes fairly easily and I feel that this would easily decompose it into
fishy trimethylamine. Also, if it is getting viscous but there is still water, when you ultimately cool it down there will still be water in your
batch of crystals. I suppose you could try vacuum filtration of this to remove as much liquid water as possible from the precipitate...
|
I used to think the same as you, but then I tried it...
The boiling does smell fishy because in the beginning, the trimethylamine that's initially in there (typical of commercial samples) boils off, but
later it doesn't smell fishy and the freshly crystallised crystals don't smell fishy at all either. It's only upon aging that the characteristic odour
develops again.
For all intent and purposes, the crystals are dry except for trace water. If you do it, you will see.
I think the viscous liquid is molten choline chloride. I didn't measure the temperature, but it was @#!%! hot at the end (gas burner on full with a
large skillet pan over the flame). Luckily I had a hunch that it was dry and so turned off the gas. I was pleasantly surprised when cooling that
everything solidified into a crystal mass.
If you want it 100% dry, just place those crystals in a desiccator or use vacuum. At least there will be very little water to have to remove at that
stage. There might be some ethylene glycol contamination, maybe the crystals can be washed with a non-polar solvent to remove that. I didn't bother.
My point... you CAN simply just boil it down, so long as you know where to stop it. Anyway, if you stop it too early and it's too 'wet' when cooled,
just turn up the heat and heat some more.
Be careful, don't leave it open for cooling, ChC is damn hygroscopic and it might liquefy again if you leave it open.
I used a thick-base pan when doing it with the idea that a larger surface area would be better for the end stage when it's evaporating through a film
more than boiling. I don't know if that's critical, just saying...
[Edited on 5-12-2015 by deltaH]
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
Interesting that you did this on a gas burner. I hope you are aware that trimethylamine is flammable? I will try this with about 50mL of solution on a
small pan. Did you slowly turn up heat or put it directly over the high flames? I can imagine something like that sending boiling ChCl solution
everywhere.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The amount of trimethylamine that comes off is very little (less than a couple percent?), it's a minor contaminant (although it smells horrible)...
and I'm still here 
[Edited on 7-12-2015 by deltaH]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I'm going to have to try that some time;my choline chloride smells awful! I thought that was just a property of that compound.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The problem is that in time it will start stinking again, one of the big problems with my choline soaps  I could make fresh stuff that smelled fine, but after a couple of weeks... I could make fresh stuff that smelled fine, but after a couple of weeks...
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
No, sadly choline chloride just likes to undergo a Hoffman degradation into trimethylamine (wonderful fishy smell), water, and ethenol/acetaldehyde
from what I can find. Mechanism picture attached, taken from Electrochemical decomposition of choline chloride based ionic liquid analogues. Given that it requires the hydroxide rather than the chloride, I
think it may be happening due to trace presence of water. I couldn't find more mechanisms on the degradation, but I wonder if there isn't one that
would occur in anhydrous ChCl. Perhaps someone should attempt to seal some choline chloride with negligible water content and leave it out for a while
to test if it produces trimethylamine.
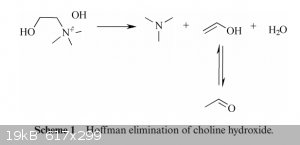
[Edited on 9-12-2015 by DeIonizedPlasma]
|
|
|
| Pages:
1
2
3
4
5 |