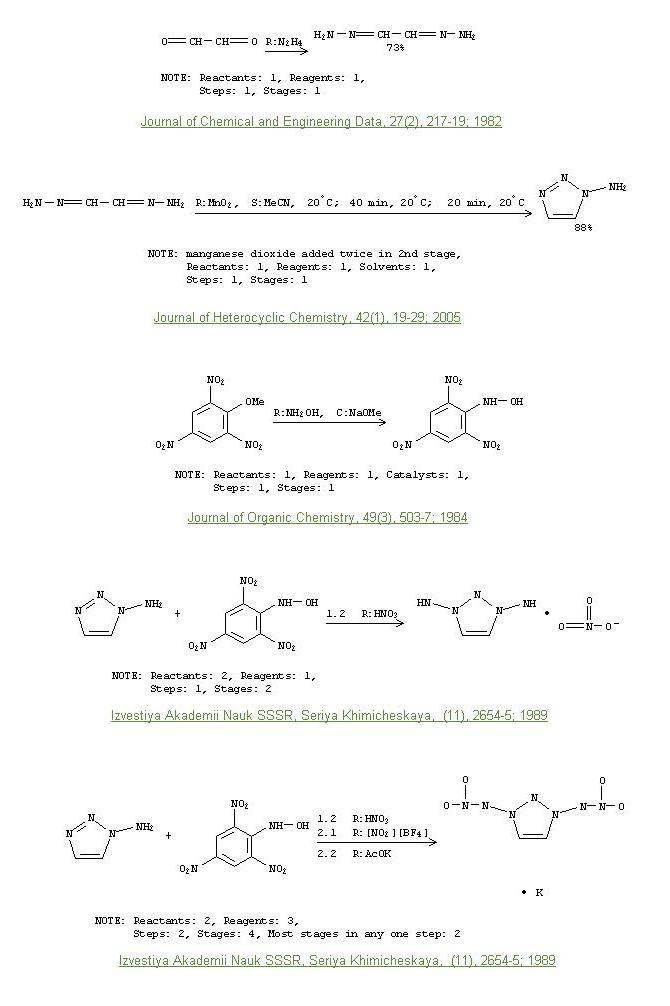
N-Nitroimides
A rather insane explosive moiety N-N-NO2. I was looking at the synthesis of what looks to be the most energetic of the known N-nitroimide compounds
(containing the substructure O2N-N-N-N-N-N-NO2  ) and its preparation looks
quite simple up until the need for a final nitronium tetrafluoroborate nitration, but it still it deseves a mention. Using HNO3 without NO2.BF4 yields
the nitrate salt. ) and its preparation looks
quite simple up until the need for a final nitronium tetrafluoroborate nitration, but it still it deseves a mention. Using HNO3 without NO2.BF4 yields
the nitrate salt.
I've attached the reaction schemes for the intermediates, the only sticking points being glyoxal (not so bad), and the NO2.BF4. The prep of
trinitroanisole has already been given in the <a href="http://www.sciencemadness.org/talk/viewthread.php?tid=8027">thread on pentryl</a>.
A few more are mentioned in Agrawal & Hodgson "Organic Chemistry of Explosives" (2007) pp. 287-288. A more comprehensive review of the
N-nitroimides is in; E. T. Apazov, S. L. Ioffe, A. V. Kalnin, Y. N. Strelenso and V. A. Tartakovsky, Mendeleev. Commun., 1991, 95. (I dont have
access).
[Edited on 21-5-2008 by Axt]
|