| Pages:
1
2 |
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
diethyl ether
I have checked out numerous procedures to make this compound, and found one that stated quiet clearly, that denatured ethanol(methylated spirits)
could be used. I decided instead to distill methylated spirits and collect gaseous products(methanol) in the range between 60 - 75 deg celcius, where
only about 10 or so ml came over in the period of a good 2 hours. Total pot contained 500ml.
Rather than go ahead with the reaction, I decided to test the addition of 98% sulphuric acid to 50ml of distillate and slowly - cautiously- added 10ml
of the sulphuric acid. The solution slowly turned redish brown and evolved a fair bit of heat.
I have found various procedures for the preparation of diethyl ether, but none of them described the colour of the pot when sulphuric acid is added to
ethanol. I am relatively new at practical chemistry and was surprised by the color change. I understand the mechanism of the reaction and have a good
clear preparation etc, but thought I should discuss the color change and question whether it could of been brought about by the use of methylated
spirits and perhaps some sort of additive that I have not removed by my initial distillation. Perhaps the color change is what just actually occurs in
this reaction - any thoughts, or answers to my questioning would be appreciated.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The color change even occurs with pure ethanol. It seems that there is a side reaction, in which sulphuric acid dehydrates the ethanol and
subsequently some polymeric compound is formed. Many times, such polymeric compounds are brown or black and in very low concentrations they tend to be
yellow, orange, red.
I have similar results with acetone.
|
|
|
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
Thanks. I just want to be careful with things and when something unexepected occurs I want to know what is happening.
bmc
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
the pot will end up BLACK after heating anyway.. i your sulfuric acid clear, or colored? In any case I don't think this can cause a problem for the
recation your product will be distuille doff in any case, and you should fractionnate it afetr washing and drying. All the junk will stay in the
initial pot.
I suppose you have read Len1's excellent piece of work on the subject in prepublication?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
One other thing, since your product is methylated spirits, your looking at anywhere from 6-50% by weight methanol.
That means your going to get a bend of dimethyl, methyl ethyl, and ethyl ethers. The problem with this is methyl ether has a boiling point of like 9
degrees, methyl ethyl somewhere in between that and ethyl ether.
Yields are going to suck unless you have one hell of a chiller.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Are you sure methylated spirit is that much methanol?
I have a 2.5L ethanol from Fisher, and the label says:
Methylated spirit industrial , 99% v/v , (74 O.P, alcohol content about 99%), pure
Seems like 99% ethanol to me. Or does the 99% actually mean alcohol as both ethanol and methanol? If yes, what does the O.P mean?
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Usually, 99% is indeed the sum of both alcohols, but I do not expect more than a few percent of methanol in the methylated spirit. I think it is
exaggerated to say that this can contain up to 50% of methanol.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I agree with woelen. In the UK...
"in the case of industrial methylated spirits, with every 95 parts by volume of spirits there shall be mixed 5 parts by volume of wood naphtha.
in the case of mineralised methylated spirits, with every 90 parts by volume of spirits there shall be mixed 9.5 parts by volume of wood naphtha and
0.5 parts by volume of crude pyridine, and to the resulting mixture there shall be added mineral naphtha (petroleum oil) in the proportion 7.5 litres
to every 2,000 litres of the mixture and synthetic organic dyestuff (methyl violet) in the proportion 3.0 grammes to every 2,000 litres of the
mixture."
...according to a government website. Wood naptha is an alternative name for methanol. It goes on to say...
"Water may be mixed with spirits before methylation or with methylated spirits, but the quantity of water added must not reduce the proportion or
quantity of denaturants in the resulting mixture below the proportions or quantities given above for the appropriate class of methylated spirits."
This implies that a simple distillation may not yeild absolute ethanol, even with an efficient column, as any water present will produce an azeotrope.
However methylated spirits is a cheap and easily accessible source of ethanol.
|
|
|
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
| Quote: | Originally posted by Klute
the pot will end up BLACK after heating anyway.. i your sulfuric acid clear, or colored? In any case I don't think this can cause a problem for the
recation your product will be distuille doff in any case, and you should fractionnate it afetr washing and drying. All the junk will stay in the
initial pot.
I suppose you have read Len1's excellent piece of work on the subject in prepublication? |
The sulfuric acid is clear and no I had not looked at the prepublication work done by Len 1. I have now and I am pleased you pointed this out. Thanks.
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
| Quote: | Originally posted by woelen
I think it is exaggerated to say that this can contain up to 50% of methanol. |
I think it may depend. I have some OTC 'Denatured alcohol' that according to the MSDS is 65-75% MeOH. This doesn't really claim to approximate
ethanol though.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
what the labeling means will depend where you are, but for the UK:
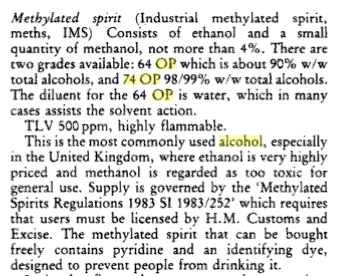
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Don't buy OTC denatured alcohol. In most major cities there will be someone that supplies solvents. There is a solvent called "Synasol, Anhydrous"
which (most of the time) consists of 95% EtOH and 5% MeOH, IPA or some other denaturant (most of the time it's MeOH). That is the minimum allowable
denaturant percentage (for MeOH) that is allowed while avoiding the liquor tax.
It's VERY cheap, too. Cheaper than buying it at the hardware store, that's for sure.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Honestly, who the heck wants to use a product laced with methanol, MIBK, and gasoline in it as a feedstock for high purity ether production?
Its fucking ridiculous how hard it is to get pure alcohol in the USA at a decent price... the closest OTC thing to pure EtOH is Everclear and that
costs $85-90 a gallon which is bullshit IMO... IIRC almost $50 of that is taxes.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Denatured alcohol here in New Zealand is called "methylated spirits", $old in supermarkets and hardware stores, avoiding the large excise duty on pure
ethanol (which could be used as booze). It has a blue dye, which I presume is methyl violet, but the labels on the 1L and 5L plastic bottles that it
comes in do not say what the denaturants in it are. I will have to write to the chemical firms that produce it to find out. Ethanol is made here by
fermentation and distillation of sugar (sucrose), whey (lactose), and wheat (starches); and methanol is mostly made from the methane in natural gas in
the Fischer-Tropsh process either for export or subsequent catalytic processing into synthetic gasoline (and can also be made by destructive
distillation of wood waste or straw).
|
|
|
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
| Quote: | Originally posted by evil_lurker
Honestly, who the heck wants to use a product laced with methanol, MIBK, and gasoline in it as a feedstock for high purity ether production?
Its fucking ridiculous how hard it is to get pure alcohol in the USA at a decent price... the closest OTC thing to pure EtOH is Everclear and that
costs $85-90 a gallon which is bullshit IMO... IIRC almost $50 of that is taxes. |
I dare say you have exhaused all avenues for a cheap 100% but just in case you or another, haven't thought of this, I'd at least mention it. French
polishing requires this product and do not require great amounts. In AUS this product at 4L (aqua label methylated spirits 1000mL/L 100% ethanol) can
be obtained, but not easily, as most places do not stock- nor do they advertise for obvious reasons. It is around $20 for 4L. I chose to do the
diethyl preparation not because I cant obtain pure EtH but because the usual methylated spirits is a lot cheaper.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
So, making Et2O via "methylated spirits" (for some reason I have never seen it called that before) is cheaper than just buying or distilling Et2O (EtH
is ethane, BTW) in your area? That's crazy.
There is a source (in the US) of 80% Et2O (with the other 20% being propane, butane and carbon dioxide and a viscous oil, all of which are much more
easily removed than heptane or hexane). In fact, this product can sometimes just be used as is. Noone ever seems to be interested in it's existence,
though. I mean, you don't even have to fractionate this stuff, just distill and you're done.
I hope I'll never have to use the H2SO4 + EtOH method because it seems like a royal pain in the ass. Good luck, though.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
I haven't given it a thorough search to obtain Et2O but it doesn't seem to be common OTC in AUS. As I stated, I am learning practical chem so I
thought preparing this compound is a good start. I have just begun simple distillation and have not yet ventured to using a fractional column. You got
to start somewhere ha!
bmc
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you want to obtain some practical experience with chemistry, then making diethyl ether is not the thing to start with for the following reasons:
1) The reaction, making the stuff, is not clean at all. There are many side-reactions.
2) Diethyl ether has a very low boiling point. You need really good cooling, plain tap water at room temperature may be insufficient.
3) Diethyl ether is EXTREMELY flammable. If you don't have practical experience, then there is a big chance of getting a major accident. A simple
electrostatic spark may cause a big explosion and subsequent fire. You need to take very good safety measures when dealing with this highly volatile
and flammable compound.
If you want to get experience and you also want to make some useful reagents, then you could try to make nice colorless pure HCl from OTC muriatic
acid, or from table salt and dilute sulphuric acid. Another nice thing to do is making pure aqueous ammonia at a decent concentration from NaOH and
ammonium sulfate fertilizer (or ammonium nitrate, or ammonium chloride). Both of these can be done nicely with a distillation setup. In the case of
ammonia, you put some water in the collector flask.
|
|
|
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
Thanks woelen, When I say I am learning prac chem, I do have a year worth of a Diploma level lab tech course and from that I am really safety
conscious. I am currently an undergraduate in Biomedical Science but lack distallation experience. I have understood your post and will be mindful and
not be in too much of a hurry in this area. So far I have made a shit load of salts via reactions including electrolysis. My initial distillation was
ethanol I prepared from yeast and sugar which fermented, which went well. I didn't get as much as I thought and will look at improved yields. I did do
the ether prep and placed the reciever flask in ice water that included salt which brought the temp down enough. I have a good column but need another
stand and do not want to make things do as I do see the importance of being set up right. Getting the distillation glassware was a buz and I suppose
Ive been in abit of a hurry to try these things out. I love doing this stuff. It is insanely good. I will also do your suggestion of concentrating
ammonia in di water.
bmc
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
I'm pretty sure when I made Et2O you had to add the EtOH to the conc sulphuric acid @ 125 C and not the acid to the EtOH
Also you need many long distillation columns filled with ice cold water
much easier to get it in other ways, and yes, colored products in the rxn flask are inevitable
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
undead_alchemist
Hazard to Others
  
Posts: 189
Registered: 12-1-2007
Location: Vancouver, Canada
Member Is Offline
Mood: Tired, Cleaning up corporate messes at work!
|
|
Pure EtOH is even harder to find here in Canada,
Have yet to find any place that sells it.
Most of what you can get is only 40%, and that is over priced
Even the Lab Suppliers don't have it, well at least listed in their catalogs.
It costs me less to buy ether then to make it.
$195 for 18L Lab Grade.
Well helps to be able to buy though my work too.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
| Quote: | | dare say you have exhaused all avenues for a cheap 100% but just in case you or another, haven't thought of this, I'd at least mention it. French
polishing requires this product and do not require great amounts. In AUS this product at 4L (aqua label methylated spirits 1000mL/L 100% ethanol) can
be obtained, but not easily, as most places do not stock- nor do they advertise for obvious reasons. It is around $20 for 4L. I chose to do the
diethyl preparation not because I cant obtain pure EtH but because the usual methylated spirits is a lot cheaper. |
If Australia's liquor laws are in any way similar to the US's then this cannot be "100% pure EtOH". It wouldn't be called "methylated spirits" if it
were just EtOH. Also, if it says "100% ethanol" that usually does not mean pure ethanol. They mean it is anhydrous but still contains MeOH or some
other denaturant. I know this for a fact as the EtOH I buy claims to be "100% EtOH, Anhydrous". At best this is misleading and at worst a flat-out
lie. It should say, "95% EtOH, 5% MeOH, Anhydrous".
ALMOST NOTHING that is pure EtOH can be sold without a liquor tax. There are VERY FEW exceptions and those can only be had by pharmaceutical
companies, some industry and some specialized labs. There will certainly not be any pure EtOH sold to individuals without a high liquor tax (at least
in the US).
In fact, I did some research a while back on the subject and apparently the ONLY way even pharm companies can buy it tax free is when they use it in
medicine. For some reason they still use denatured for synthesis. The conclusion I drew from that is that the govt. is so restrictive that the ONLY
way they allow EtOH to be untaxed is if it shows up in a drug end product. Another reason could be that denatured is cheaper and most syntheses will
not be hurt by 5% MeOH.
Either way it's bullshit, IMO. These laws date back at least a hundred years and are out of date, IMO.
Although I'm sure a decent chunk of our govt's money comes from taxation of liquor. It's a double edged sword. Perhaps... Nevermind, don't want to
get into that!
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Siddy
Hazard to Self
 
Posts: 81
Registered: 8-10-2007
Member Is Offline
Mood: No Mood
|
|
Thats true MagicJigPipe, even people in industry, such as cleaners pay over $80USD for anhydrous EtOH -its only labs that get it for $2/L, and yes
sometimes even labs will used denatured just so there lab report has one less potential drug listed that week.
I don't have any issues with the high taxes, in countries like USA, AUS etc where drinking is so big, the govt makes a lot of money which means they
don't need to tax other things so much - works out for the non-drinker (or atleast non-alcoholic). Although it doesn't stop the govt from becoming
greedy and just taxing everything like crazy...
Just thought id add, a lot of "methylated spirits" no longer contain methanol, they use less toxic (but worst tasting) denaturing agents, such as
acetates. These usually cost a dollar or 2 more than the methanol ones. 2 out of 3 local brands have done this where i live.
Brew, i think you can make it out of Ethylene glycol, found in car coolant (antifreeze), but EtOH is the easiest. Also, i doubt its illegal for you to
import 10 odd bottles of Methylated spirits.
[Edited on 12-6-2008 by Siddy]
|
|
|
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
I was somewhat shocked to find this product and was told that it is free of methanol or other denaturants, as the process of shellacking requires 100%
purity. I showed the person my Uni card -photo Id, and said I am doing various experiments at home associated with my degree, and need to know whether
they felt confident with this 100% purity status of this product and do they have total confidence with the producers claim etc. Once again they
stated that they did. I am not drinking the stuff, nor am I breathing in its fumes and at this stage have not needed ethanol at such purity. I
stumbled upon this outlet, that mainly deals with tradesman and did so as I was looking to buy toluene. I have never seen this aqua labeled methylated
spirits before, nor have I seen it in other shops. It is quite a well known brand name in Aus. I will check its b.p. My scales are not good enough to
distinguish between ethanol and methanol if this is the case- specific gravity etc. I will also contact the producers and state that one of my
shellacking buddies has said that it does contain a denaturing compound etc, and see what they say. I am interested now that you have questioned this.
I will post what I find.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Get some sugar, 48hour turbo yeast, and build yourself one of these:

Then stick a column on top of it and a reflux dildo:


Presto cadabra, 90% pure EtOH after 2 distillations. Run the EtOH again thru a column with molecular sieves, then redistill... there is your EtOH...
not that much work (just a lot of babysitting) and still less than $25 per gallon.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
| Pages:
1
2 |