| Pages:
1
2 |
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
How long do you ferment your mixture, evil_lurker? I've left a sugar and yeast mixture to sit for over 1 week, is that too much?
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
With turbos, 3 or 4 days tops... they are usually done by then. Personally I ferment about 30-32lbs of sugar in one lick with a total volume of 15
gallons at a time using two packs of turbo. Not supposed to do this but it works. It helps immensly to put a mag stirrer under the fermenter and a fan
blowing on the outside as a lot of heat is generated by the yeast.
Another equally effective method is to run a partial corn/sugar wash. Over on homedistiller.org they have a method called UJSM (Uncle Jesse's Simple
Mash) which basically the corn provides the nutrients for the yeast and a bit of flavor while sugar is used for the alcohol production. Unfortunately
most of the corn is not used due to the starches not being converted so to counter that if one were to convert the starches using enzymes, say maybe
10lbs worth of cracked grain and 20lbs of sugar per 15 gallons, it should be possible to do a whole batch very little cash as the corn would provide
the vast majority of nutrients for the yeast... one could just use plain old distillers yeast (500g for $15) and just add a little bit of DAP in there
for energy and phosphates.
Yeast will not ferment a plain old sugar water mixture as there are no nutrients. Bakers yeast will also not ferment to more than 8-10% alcohol
content before crapping out. One must also adjust the initial specific gravity below about 1.10 otherwise the yeast will die from osmotic pressure in
tiny little explosions.
There is a whole lot to alcohol production, but once one gets the gist of things its not to difficult. I've pretty much gotten things worked out, just
don't have a very good reflux column... once you get into the 90%+ range EtOH is very hard to fractionate to the azeotrope since the alcohol content
in the pot is constantly dropping and the reflux ratio must constantly be increased.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Thanks for the response. I had used a mixture of 25 g sugar and 25 g Brewer's yeast in 250 mL H2O, and let it sit in a jar (not airtight) for over 1
week. I then later attempted to distill at 80 deg. and didn't get any EtOH. So, nutrients is what it was missing then. It looks like 90%+ alcohol
still will likley not work for anhydrous ether (at least not directly especially if ether is volatile with water vapors), but I guess there you have
to ask yourself if water or the other contaminants are more preferable.
[Edited on 14-6-2008 by Schockwave]
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Keep in mind that the conversion of sugar to EtOH is only 42% or effective at best... the rest goes out as CO2.
If you want ether, you reallyneed 98% sulfuric and 96%+ EtOH. Mix 50-50 by volume to approx 1/2 the volume of the reaction flask. Be sure to
mix sloooowly due to heat formation... if your not careful the mixing alone with form some Et2O. Once its mixed continue heating. Et2O will start to
come off when the EtOH/H2SO4 mixture hits around 115ºC since your dealing with very dry reagents to begin with. At that point start adding in more
EtOH.
The trick is to use a packed hempel columm at least 300mm (preferably a little bit longer), magnetic stirrer, and add in the EtOH at a rate where the
top of the column stays warm (around the boiling point of ether).
What'll happen is most of the unreacted EtOH will reflux back down into the flask where it gets dehydrated, while the Et2O vapors will come right on
over due to Et2O's high partial vapor pressure.
The procedure scales up nicely with multi-liter quantities possible provided the apparatus is large enough and sufficiant cooling is available.
Its really a good procedure, even for a beginner IMO as long as the vapors are contained.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
as a source of EtOH i buy surgical spirits wich is available readily where i live.
It costs about £4 a litre though but the label says 89%EtOH and the rest is bits ad bobbs like glycerine menthol ect... they are not allowed to sell
it to under 18's wich is a good thing.
most of all my tests suggest no methanol- perfect for distilling nearly pure EtOH 
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I have recently found that diethyl ether is highly soluble in concentrated hydrochloric acid. According to this up to 150ml ether per 100ml of cold 32% HCl. It so happens that I was just contemplating the cheap/easy purification of OTC starting fluid..
Why couldn't you simply mix the starting fluid (~30% ether in heptane) with cold hardware store muriatic acid to extract most of the ether? Then pour
off the heptane. To get the ether out of the the acid, you could warm it, dilute it, and/or neutralize it. This method seems to be much easier that
fractional distillation.. and it should give a product free of heptane?
|
|
|
HydroCarbon
Hazard to Self
 
Posts: 77
Registered: 7-7-2008
Location: Anytown, USA
Member Is Offline
Mood: No Mood
|
|
On the note of ethanol distillation: this experiment walkthrough may be helpful. It has a nice standard curve of density vs. mass% ethanol, which can
be helpful in determining the concentration of your product (assuming little to no contamination).
http://www.cerlabs.com/experiments/10875407404.pdf
We did this in my organic lab for school not too long ago and got to 87.5% ethanol by mass easily.
If you need the ethanol you could always make it according to that method in small batches with standard lab equipment. I'm sure there are countless
substitutes for the energy/nutrient requirement other than disodium hydrogen phosphate, if you cant obtain that. Carbon filtration will also be
necessary as our product had a yeasty aroma, indicating some volatile contamination from the yeast.
As evil_lurker already noted you probably want as pure ethanol as you can get for this reaction due to the fact you make 3 different ethers with a
me/etOh mixture.
You may already know, but don't let the ether sit around for too long. I'm not sure if these warnings are over stated or not; but ethers can
auto-oxidize in air to form explosive shock sensitive hydroperoxides. This also goes with warning about the extreme flammability and volatility of
ether. When using ether even at room temperature you can see vapors mixing in air when pouring. These could easily be set off by a spark from a
nearby electrical device such as a hot plate.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  | I have recently found that diethyl ether is highly soluble in concentrated hydrochloric acid. According to this up to 150ml ether per 100ml of cold 32% HCl. It so happens that I was just contemplating the cheap/easy purification of OTC starting fluid..
Why couldn't you simply mix the starting fluid (~30% ether in heptane) with cold hardware store muriatic acid to extract most of the ether? Then pour
off the heptane. To get the ether out of the the acid, you could warm it, dilute it, and/or neutralize it. This method seems to be much easier that
fractional distillation.. and it should give a product free of heptane? |
497 all I could get from that reference was useless 'snippet views'.
Did you go ahead and test your theroy?I would be interested in results.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
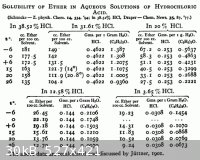
The source, Seidal's Solubilities, is public domain and can be downloaded from the Internet Archives.
Note that 20% hydrochloric acid is nearly the constant boiling composition, the highest boiling point mixture. Anything more concentrated than that
will give off HCl gas when heated. You might extract with hardware store grade 36% and add water to dilute it to 20%, then distill the Et2O out of
that, and finish by distilling the 20.2% acid for lab use.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I've been trolling through the old ether posts for some inspiration and information on the finer details.
D9 is correct that, in the UK, there is a very specific set of rules regarding the composition of methylated spirits.
| Quote: | | Completely denatured alcohol must be made in accordance with the following formulation: with every 90 parts by volume of alcohol mix 9.5 parts by
volume of wood naphtha or a substitute for wood naphtha and 0.5 parts by volume of crude pyridine, and to the resulting mixture add mineral naphtha
(petroleum oil) in the proportion of 3.75 litres to every 1000 litres of the mixture and synthetic organic dyestuff (methyl violet) in the proportion
of 1.5 grams to every 1000 litres of the mixture. |
Lurker, I absolutely loved your brew it at home attitude and method, particularly the reflux dildo and keg. I also love the fact you're using turbo.
If anyone is ever planning to do some brewing for distillation, forget the wine shops and supermarket, buy a pack of distillers turbo
(partyman.se sells it online). No questions askable, it's incredible. Massive alcohol yield by comparison and it runs so fast it looks like it's going
to kill it's self within a day or two.
I have a 10l flask of it brewing now. I could just buy alcohol off the shelf, but that's no fun. I find it really interesting, the idea of going from
sugar and yeast to glassware synthesis.
The water / ethanol azeotrope breaks somewhere around 75mBar I believe, meaning you can yield 100% pure alcohol under vacuum.
I certainly don't want any explosive rubbish forming when I'm doing it. I think I may piranha bang all of the glass with 35% H2O2 first, and I have
about a kilo of hydroquinone lying around, waiting for a job, which (from my quick read thus far) would do well for the hot flask in terms of keeping
it unexploded.
[Edited on 28-8-2010 by peach]
|
|
|
| Pages:
1
2 |