Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Dinitrotyrosine
Dinitrotyrosine is made by the nitration of nitrotyrosine nitric acid and it is said to be very stable, and forms a ruby-red barium salt
(Ba.C9H7N3O7 + 2 H2O) which is said to explode violently when heated.
Nitrotyrosine nitric acid is made as detailed by Strecker in A. 73, 70 by solubilizing tyrosine in aqueous nitric acid. Preparation is described in A.
116, 77 as follows: where 1 part tyrosine has 4 parts water poured over it, and then eventually 4 parts of HNO3 (sp. gr. = 1.3) is added. After
standing for 12 hours in the cold, the crystals are removed and then added to so much NH3 until no red color occurs. They are pale yellow-verruciform
needles which are very slightly soluble in cold water, unsoluble in alcohol and ether but solubilize in alkalis with a red coloring, and easily
soluble in dilute mineral acids. It also forms salts.
Dinitrotyrosine (C9H9N3O7 = C9H9(NO2)2NO3) is prepared by the evaporation nitrotyrosine nitric acid with the same parts by weight of water and HNO3 (d
= 1.3) in gentle heating (A. 116, 82; Z. 1869, 669). It is gold-yellow leaflets (from water) which are very slightly soluble in water, and barely
soluble in hot alcohol it is said to also not bind with acids. Where A = Annalen der Chemie and Z = Zeitschrift für Chemie.
Beilstein 2, 1007
Beilstein 2, 1008
[Edited on 28-5-2008 by Schockwave]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here reference in English.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
The salts of 3,5-Dinitrotyrosine itself don't loolk to be of great interest, or at least of no greater interest then more easily available
dinitrophenol analogues. Tyrosine is $595 for 5kg here as a protein suppliment.
Though it may have some novel value by making use of the primary amine group such as by halogenation, its also amphoteric so nitrate and perchlorate
salts might be possible. Looks like quite a weak base though its HCl salt is given in JACS, (1915), 37, 2164-70. Though this nitration is an ugly
one, due to the difficulty in extracting it from the acids since its sorming soluble salts.
<b>3,5-Dinitrotyrosine</b> - First, an acid mixture is prepared by combining 22 g. of pure concentrated sulfuric acid and 3 g. of
concentrated nitric acid. This mixture is then cooled to 10°, and 2 g. of finely pulverized tyrosine added slowly in small portions while the
solution was constantly stirred and the temperature kept below 10°. It is not advisable to nitrate more than 2 g. of tyrosine at a time and these
should be added to the acid as rapidly as possible. A complete solution of the tyrosine is not effected by the operation and after final addition of
the acid the mixture is then poured immediately into water.
This operation was repeated six times and the combined solutions worked for dinitrotyrosine. In order to isolate the free acid, the sulfuric acid is
exactly precipitated as barium sulfate and the barium free filtrate then made distinctly alkaline with ammonia and the resulting solution finally
evaporated to dryness, by heating at 60' under diminished pressure. On treating the resulting residue with about 25 cc. of cold water the
dinitrotyrosine is obtained in the form of a bright red ammonium salt moderately soluble in water. In order to obtain the free acid this salt is
decomposed with a little dilute hydrochloric acid and the dinitrotyrosine dissolved in boiling water. It is well to digest with bone-coal here to
clarify the solution. On cooling, the dinitrotyrosine crystallizes in beautiful, goldenyellow plates containing water of crystallization.
From 12 g. of tyrosine we obtained 6 g. of pure dinitrotyrosine. Especially characteristic is the behavior of this acid when heated. When heated at
140-150' it loses its water of crystallization and assumes a-brick-red color. At 220-230° it decomposes with effervescence. The acid does not lose
its water of crystallization when heated at 110°.
<b>The Hydrochloride of Dinitrotyrosine</b> - C7H9O8N3.HCl.-This salt is prepared by dissolving dinitrotyrosine in hydrochloric acid and
then adding to the solution strong hydrochloric acid. It separates in the form of bright yellow plates which begin to darken at 220°, when heated in
a capillary tube, and decompose at 230°. with effervescence. The salt dissolves in water and is precipitated by dilution with concentrated
hydrochloric acid. The hydrochloride of o-nitrotyrosine is characterized by similar properties. For analysis, the salt was dried at 110°.
<b>Ammonium Salt of Dinitrotyrosine</b> - This is prepared by dissolving the acid in a small volpme of warm ammonia solution and then
diluting this with strong aqueous ammonia. On allowing the solution to cool the salt finally deposits as beautiful, red prisms. The salt decomposed
when heated above 230'. It was dried for analysis at 110°.
[Edited on 29-5-2008 by Axt]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Thanks for the information. I think you're right it about the energetic properties, considering the oxygen content it could be weaker than
dinitrophenol salts. The reason it interested me is because aside simple sugars, this is the only supplement known to me which can be nitrated to
yield such compounds. A cheaper and more powerful idea is from the nitration of PABA. In Beilstein it is mentioned that if 3-amidobenzoic acid is
nitrated with fuming HNO3 (process described in Annalen 139, 11) then trinitrooxybenzoic acid results, at least the barium salt of which is said to be
very explosive. PABA is 4-aminobenzoic acid:
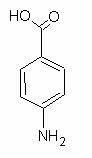
An analogous nitration of this also likley yields a trinitrooxybenzoic acid.
Beilstein 2, 972
[Edited on 29-5-2008 by Schockwave]
|
|
|
|