Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
1,3,3-Trinitroazetidine (TNAZ)
US5336784 (http://www.pat2pdf.org/patents/pat5336784.pdf) to the univ of California appears to be the earliest patented process for preparing TNAZ. Parts of
the description are poorly written & some yields are not given. While it states that it is an improvement over earlier published methods (which
had 10% overall yields), this one doesn't appear to be any improvement over that.
[Edited on 27-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
TNAZ has some good properties like high VOD (8.6 km/s), detonation pressure, and power but for a potential melt-cast replacement for TNT it has been
deemed unsuitable based on things like difficulty of synthesis, its volatility, and sensitivity. Report: http://dspace.dsto.defence.gov.au/dspace/bitstream/1947/3956...
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Schockwave
TNAZ has some good properties like high VOD (8.6 km/s), detonation pressure, and power but for a potential melt-cast replacement for TNT it has been
deemed unsuitable based on things like difficulty of synthesis, its volatility, and sensitivity. |
Thanks. This academic synthesis in that patent didn't look very encouraging. Even the later patents look like industrial dead-ends.
[Edited on 27-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
AH-Poster
Harmless

Posts: 9
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
TNAZ synthesis
Trinitroazetidine (TNAZ) is unique in that it is based on a square ring. The strain from this configuration, as well as the high density from this
geometric structure, lends increased power to the compound. It has melting point of 103C, density of 1.84 g/mL and thermal stability of up to 240C.
The main difficulty of the synthesis is initial formation of the square azetidine precursor, which deals with some advanced organic chemistry, so the
posters in the organic section might be able to help out.
Below, four different routes for the synthesis of TNAZ are presented, along with several relevant precursors. This compound and its precursors require
a very large quantity of steps. Doing just one of them a little off will throw everything else off, so this is more for information purposes, than an
actual guide. Also, please take everything with a big grain of NaCl. This is more of a scaffold for planning a synthesis, and there are several gaps
that need to be filled in. Apologies, things are a bit disorganized.
Synthesis 1:
CH2=CHCH2Cl + HOCl → HOCH2CHClCH2Cl mixed with ClCH2CH(OH)CH2Cl
The resulting mixture of alcohols is then treated with NaOH to form epichlorohydrin (with NaCl byproduct).
Epichlorohydrin is an epoxide of propanane with a chlorine atom.
Tert-butylamine, ( formula [CH3 ] 3CNH2 ) and epichlorohydrin react to form 1-tert-butyl-3-hydroxyazetidine.
The 1-tert-butyl-3-hydroxyazetidine is then treated with Methanesulfonyl chloride CH3SO2Cl which forms an ester on the 3-position. This ester is then
reacted with sodium nitrite, with a small ammount of 1,3,5-Benzenetriol (which is both the symetric tri-ketone of hexane, and tri-hydroxy benzene,
because of tautomers) present. This forms 1-tert-butyl, 3-nitro azetidine. The 1,3,5-Benzenetriol helps to prevent nitrite esters from forming.
Sodium nitrite and sodium persulfate, with sodium ferricyanide present, oxidizes the mono-nitro to the di-nitro in 60% yield. Finally, the tert-butyl
group is hydrolysed/oxidized off using acetic anhydride and nitric acid (in similar concentrations used for making other nitramines). This now leaves
TNAZ. The net procedure gives a 17% yield. Most of the inefficiency comes during the formation of the square ring and then the reaction with nitrite.
Other amines beside tert-butyl amine can probably be used instead, but likely will give lower yields.
Synthesis 2:
condensation of tris(hydroxymethyl)nitromethane with tert-butylamine and formaldehyde forms 3-tert-butyl-5-hydroxymethyl5-nitrotetrahydro-1,3-oxazine.
This is treated with HCl acid solution to yield 2-tert-butylaminomethyl-2-nitro-1,3-propanediol hydrochloride which was cyclized (using Diethyl
azodicarboxylate with triphenylphosphine which initiated a Mitsunobu reaction) to 1-tert-butyl-3-hydroxymethyl,3-nitroazetidine hydrochloride. The
reaction is conducted at 0degC, and the ether solution of DE-azodicarboxylate added last, where it is very slowly added with stirring. This is allowed
to warm to room temperature for 2 hours. Note that the DE-azodicarboxylate gets reduced during the reaction, and the Ph3P gets oxidized.
This was then treated with sodium hydroxide and then nitrated (which also oxidized the molecule) to give 1-tert-butyl-3,3-dinitroazetidine. This was
then reacted with NH4NO3 and acetic anhydride to give TNAZ. Based on the starting reactants in the procedure, this gives a 55% yield.
Alternatively,1-tert-butyl-3,3-dinitroazetidine reacts with benzyl chloroformate to yield 1-(benzyloxycarbonyl)-3,3-dinitroazetidine. The
Benzyloxycarbonyl group has a structure of (C6H5)CH2OC(=O)R, where R would, in this case,
be the Nitrogen atom on the dinitroazetidine ring. This Benzyloxycarbonyl group can easily be hydrolyzed off the square ring with NH4OH, leaving
3,3-dinitroazetidine.
If an acetyl group is on the nitrogen atom in the ring, this can be converted to the N-nitro by nitrating with 98% nitric acid, or using ammonium
nitrate in acetic anhydride.
Oxidixing agents, such as pyridinium chlorochromate, can oxidize the hydroxyl group (in the 3-position on the ring) to the ketone, leaving the ring
intact. The ketone of azetidine is known as azetidinone. From here, the oxime can be formed, and then a dinitro formed by bubbling in NO2, then
treating with H2O2.
Synthesis 3:
CH2=CHCH2NH2 can be treated with Br2 and Et2O at 15C to form HBr.(NHC2H3)CH2Br. The group (NHC2H3) is a triangular ring, the CH2Br group is attached
to a carbon atom on the ring, and the amine part of the ring has formed a hydrobromide salt.
Alternatively CH2=CHCH2NH2 can instead react with SO2Cl2 and CH2Cl2, by being heated, to form HCl.(NHC2H3)CH2Cl, which is the same compound above,
except with chlorine replacing the bromine atoms.
The HCl.(NHC2H3)CH2Cl (or the one with bromine instead), thus formed is reacted with BuLi and THF at (minus) -78C to form "azabicyclo" propane, which
is basically two triangles connected together, forming a square, with a nitrogen atom in the corner connected to all three carbon atoms. This is an
unusual structure, and obviously the molecule is very strained.
Treatment of this molecule with formic acid and THF, then with HCl and methanol will form 3-hydroxy azetidine hydrochloride,
which has a formula of HCl.(NHC3H5)OH, where the group (NHC3H5) is a square ring. A group on the nitrogen would be considered in the 1-position,
whereas a group on the carbon atom on opposite end of the ring would be in the 3-position. The hydrochloride salt converts to plain 3-hydroxy
azetidine when it is neutralized by a weak base.
THF is just a type of cyclic ether. Regular ethyl ether can be used instead. BuLi can be prepared by reaction 1-bromo-butane with lithium metal, using
a solvent such as Et2O or benzene. If 1-chloro-butane is used instead, a precipitate of LiCl will form, since this salt does not form a complex with
the BuLi like LiBr does. Also 1-chloro propane could probably work instead.
(1-chloro propane can be formed by reacting acetone and a limited quantity of Cl2 at room temperature, forming chlroacetone, then reducing this ketone
by using anhydrous Hydrogen Iodide). Note that ethers such as THF react with BuLi above (minus) -20degC, therefore the temperature must be kept below
this the whole time. Dry ice with acetone "ice baths" achieve a -78C temperature.
As a side note, when BuLi is heated, it forms lithium hydride and gives of Butylene gas C4H8, obviously this is in the absence of air.
Diethyl azodicarboxylate is a reddish orange liquid, with a structure of CH3CH2OC(=O)N=NC(=O)OCH2CH3
Hydrazine condenses with ethyl chloroformate to form the the above compound, except with two hydrogen atoms on the two central nitrogen atoms. In the
reaction, hydrazine is gradually added in small additions, and after each addtion, Na2CO3 is added to neutralize the resulting HCl that gets formed.
The central hydrazine group --NHNH-- can be oxidized to the diazo group --N=N-- using chlorine to form the final diethyl azodicarboxylate. This
chemical gives off very poisonous fumes, which is suggested by its acronym, DEAD, which is what you may be if you do not take proper precautions!
Ethyl chloroformate can (probably) be formed by reacting chlorine with the ester of ethanol and formic acid. Ethyl formate smells somewhat like rum,
and this ether is not too poisonous. Ethers can be prepared by distilling the alcohol and carboxyl- containing acid with fairly concentrated sulfuric
acid, although too strong a concentration may further dehydrate the ether.
An unusual reaction is: the Trioxane form of formaldehyde disproportionates, using a boric acid catalyst, to form methyl formate. Trioxane was heated
with a minute quantity of boric acid at 250C for several hours, using a hydrocarbon solvent. This gave a 70% yield of methyl formate. If a sodium
ethoxide catalyst is used instead, the mixture does not require heating.
"Boric acid catalyzed Tishchenko reactions", by Paul Stapp, Journal of Organic Chem.(1973)
The methyl formate can substitute for the ethyl formate, when making the azodicarboxylate ether, Dimethyl azodicarboxylate being formed instead.
3-Chloro Propylene
this compound is difficult to prepare, one way is to pass chlorine gas, preheated to 400C, and react it with propylene gas, which is the product of
isopropyl alcohol with concentrated H2SO4. If the chlorine gas has been preheated, the main reaction will be the formation of 3-Chloro Propylen,
through a radical mechanism. Otherwise chlorine will just react to form the undesirable byproduct of 1-chloro propylene ClCH=CHCH3. This undesirable
reaction even happens when the two a simply mixed without heating. A longer route exists is it is desired to work with lower temperatures.
Synthesis of 3-hydroxy propylene CH2=CHCH2OH
A mixture of 500g anhydrous oxalic acid and 500g of glycerol was heated under reduced pressure, using a hot water bath for at least 4 hours until
formic acid ceases to distill over. The mixture was then gradually heated to 240degC (under normal pressure) the flask being fitted with a
fractionating column. At 220-225degC carbon dioxide is given off and a mixture of approximately equal amounts of 3-hydroxy propylene and allyl formate
distilled, leaving a residue in the distillation flask containing about half of the glycerol initially used. only minute traces of acrolein is
produced in the reaction. The distillate was treated with 50g NaOH in 1L water to hydrolyze the formate). This is distilled after waiting 6 hours. The
first 300 mL of distillate contained all the allyl alcohol, which after fractionation yielded 200g of a allyl alcohol/water mixture (bp 87-88C) which
may be dehydrated using dry K2CO3, yielding approximately 150g of anhydrous 3-hydroxy propylene.
The glycerol residue left can be resused if the procedure is repeated. The yield of allyl alcohol is nearly quantitative calculated on the amount of
glycerol reacted. Note that formic acid is produced in the reaction. Alternatively, glycerin and formic acid may be used to make 3-hydroxy propylene
instead, see the "organic precursors 2" section.
3-hydroxy propylene may be converted into 3-chloro propylene, which can then be used in the TNAZ synthesis. This may be accomplished using acetyl
chloride CH3C(=O)Cl, which can be prepared by reacting glacial acetic acid with SCl2.
SCl2 from buring sulfur in chlorine gas that has been dried by passing through baked CaCl2. Note that SCl2 reacts with water to form SO2, S, and HCl
gas.
Acetyl Bromide could be used instead, forming 3-bromo ethylene, which can then react with anhydrous ammonia to form CH2=CHCH2NH2, with a NH4Br
byproduct. This could be used as the starting precursor in synthesis 3.
Synthesis 4:
NH2C(CH2OH)3 was added to acetic acid using a chloroform and ethyl ether solvent, then anhydrous HBr and glacial acetic acid were added. The mixture
was put into a sealed tube and heated to 155degC. This formed HBr.NH2C(CH2Br)3 in a 70% yield. This was then reacted with sodium hydroxide at 80degC
under reduced pressure (10% of regular atmospheric). This formed BrCH2C{CH2}2N, where both the {CH2} groups, as well as the 3rd Carbon atom in the
sequence are bonded to the Nitrogen atom. This is a "azabicyclo" compound again. It basically looks like two triangles put together to form a
parallelogram. This is reacted with a solution of sodium nitrite and a solution of HCl to form 1-(NO),3-(NO2),3-(CH2Br)-azetidine, but the yield is
only 10%. The structure can also be shown as O=N(NC3H2)(CH2Br)(NO2), where the last two groups are bonded to the same carbon on the opposite end of
the square from the nitrogen atom. This compound has a nitrosamine group in it. This is then oxidized with nitric acid (optionally using
trifluoroacetic acid solvent) at 0degC, then neutralized with boiling bicarbonate solution, and finally reacted with an alkaline solution of sodium
nitrite with ferricyanide and persulfate. The compound formed is now TNAZ. This whole procedure gives very low yields, and so synthesis 4 is not
suitable for making more than an extremely small quantity of TNAZ for studying. One added note, the neutralization with bicarbonate is very slow since
the the intermediate ring compound is not soluble in water. Sodium Iodide forms a complex with the common solvent DMSO, and once the sodium ions are
thus dissolved, sodium bicarbonate will also be sparingly soluble in the DMSO-NaI mixture. This complex is discussed in one of the "under
construction" sections. This side step is extra trouble, but allows the neutralization to procede rapidly without trouble.
|
|
|
AH-Poster
Harmless

Posts: 9
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
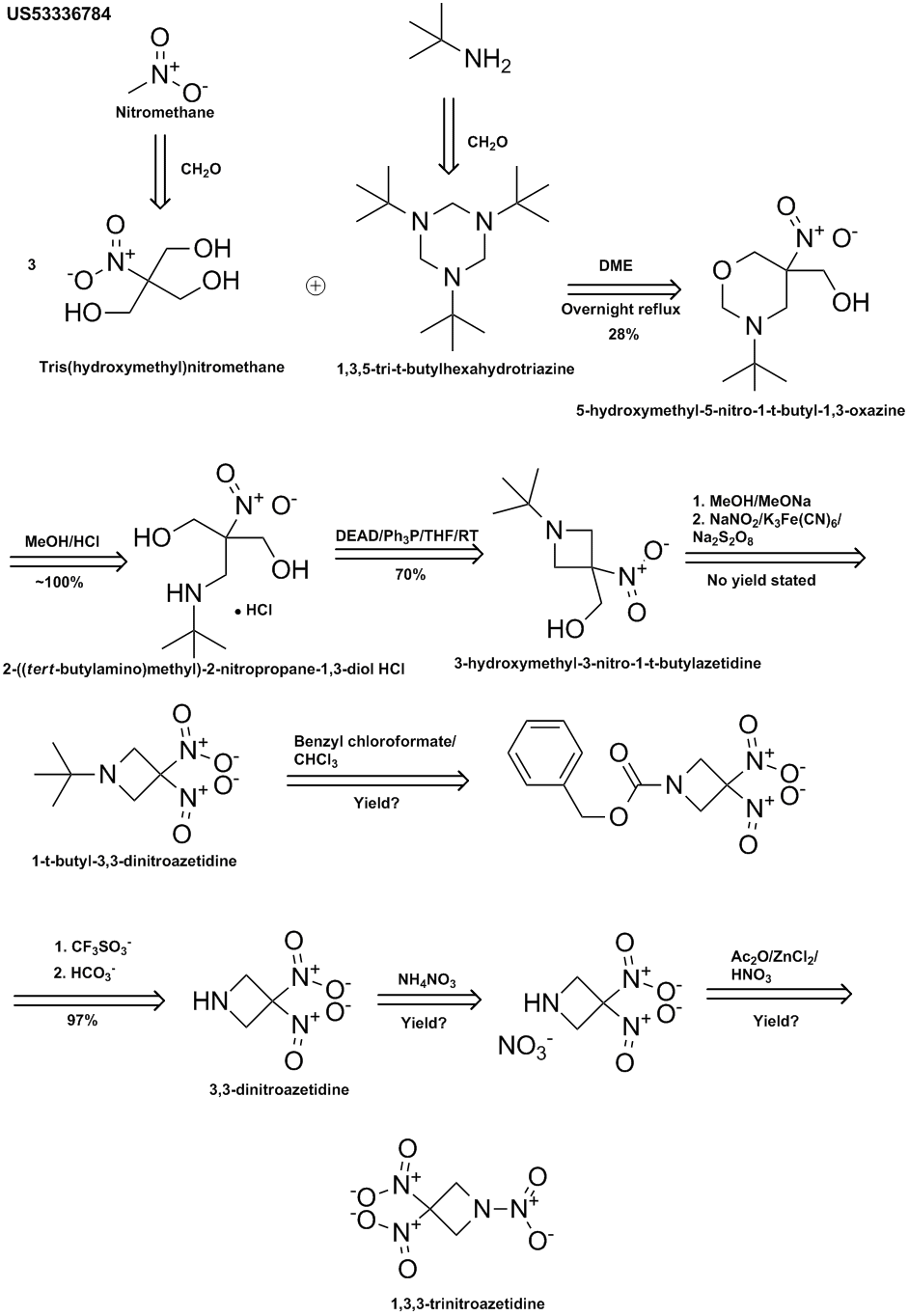
Something else relevent to making the 3-chloro propylene above, which is also known as "allyl chloride",
"Acetyl chloride-ethanol brings about a remarkably efficient conversion of allyl acetates into allyl chlorides"
Veejendra K. Yadav
This is in 96% yield.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
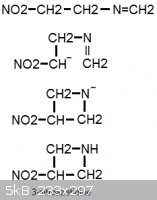
Quick idea for a much shorter route for making TNAZ:
Condense 1-nitro-2-aminoethane, NH2-CH2-CH2-NO2, with one equivalent of formaldehyde (in the form of trioxane). Then react with a non-nucleophilic
base (ideally "proton sponge"). The nitroalkane should be able to condense with the -N=CH2 group that exists in equilibrium.
O2N-CH[-]CH2-N=CH2
This should hopefully form 3-nitroazetidine, which could then easily be nitrated directly to TNAZ.
One potential problem is that vicinal amino-nitroalkanes, such as 1-nitro-2-aminoethane, are not the most chemically stable compounds,
http://www.sciencemadness.org/talk/viewthread.php?tid=16118
Formaldehyde also is prone to undergo the Canizarro reaction under alkaline conditions, but usually this effect does not predominate. For example,
2-nitroethanol can still be prepared from nitroethane and formaldehyde under alkaline conditions. And pentathyritol is made through the condensation
of acetaldehyde with formaldehyde with base. In other words, CH2O will usually condense with something else before it disproportionates.
But this could possibly be a much more direct synthesis route to TNAZ. If someone who is more knowledgable in organic chemistry could comment on this
idea...
Another distantly related cylisation reaction is
"trans-1.2-Dinitrocyclopropane (DNCP)", Wade et al. , discussed in this thread: http://www.sciencemadness.org/talk/viewthread.php?tid=18478
[Edited on 24-2-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Formatik  | | TNAZ has some good properties like high VOD (8.6 km/s), detonation pressure, and power but for a potential melt-cast replacement for TNT it has been
deemed unsuitable based on things like difficulty of synthesis, its volatility, and sensitivity. |
Actually, TNAZ has a relatively low sensitivity considering how powerful it is. TNAZ is more resistant to shock and impact than HMX, but the
sensitivity is highly variable depending on the crystal grain size. Smaller grains (5.5 micrometers) have a 66cm sensitivity using the drop height
test, while larger grains approach the 23cm sensitivity of HMX. TNAZ has a shock sensitivity of 4.3 kbar.
TNAZ has a detonation velocity between 8.6 and 8.85 km/sec, and generates a pressure of 372 kbar. One measured value for the detonation velocity was
8.73 km/sec. "Characterisation of the Sensitivity and Performance Properties of 1,3,3-Trinitroazetidine (TNAZ)" Aubert, S.A. (1994). TNAZ has
7.7% (or about 8%) more explosive power per unit of weight than HMX. (HMX has a higher detonation velocity of 9.1 km/sec because it has a higher
density at 1.91 g/mL).
I do not think TNAZ has any "volatility" problems. I have done much reading/research about it, and have never read anything about this.
[Edited on 26-2-2012 by AndersHoveland]
|
|
|
|