| Pages:
1
..
3
4
5
6
7
..
24 |
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Sorry, starting from bleach just isn't worth it.
And my 5% old bleach contains way too much NaCl for strenghtening to be convenient. A lot of NaCl crystallizes out after addition of NaOH. The double
amount would crystallize after strenghtening and turn the whole thing into slush.
And do you really think the bleach manufacturers use distilled water for their product? I don't.
My stirring rod was a bit brown on its side because I stirred a MnO2 suspension with it before. The yield was still quite high, as you've seen.
What was the purpose of the gelatin, after all?
I think the catalytic action of a hundreth ppm of metal ions is only true for the un- gelatinized reaction mix.
Metal ions still have to be avoided, but the reaction isn't hyper- sensitive.
To make my 10% NaOCl, I will have to chlorinate the NaOH until saturation, and then quickly add some extra NaOH (until the chlorine smell disappears)
to stabilize the NaOCl. I hope this works.
To produce chlorine from TCCA, I simply add dilute (15- 20%) HCl. The reaction is smooth and steady (it can be controlled by warming or cooling,
warming is usually necessary). Not only the chlorine of the TCCA, but also the chlorine of the HCl is converted to Cl2 gas, making this a very
effective method.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by garage chemist
Sorry, starting from bleach just isn't worth it.
And my 5% old bleach contains way too much NaCl for strenghtening to be convenient. A lot of NaCl crystallizes out after addition of NaOH. The double
amount would crystallize after strenghtening and turn the whole thing into slush. |
That is some really strange bleach . Are you speaking of the old 12% which has deteriorated ? Because that is the only way I can make sense of the
untypical and disproportionate salt content .
| Quote: |
And do you really think the bleach manufacturers use distilled water for their product? I don't. |
The water requirement is stringent for a stable commercial product . The half-life for regular bleach should be a year or more , and to get that
requires highly pure water , if not distilled then exhaustively de-ionized . Often a second
flocculant like Mg(OH)2 is used , even done repeatedly if needed on the finished
bleach to remove any impurites which were introduced with the NaOH or from the equipment and holding tanks , as a finish treatment before bottling and
shipment . Quality control is tight , or the product doesn't keep and comes back as defective .
| Quote: |
My stirring rod was a bit brown on its side because I stirred a MnO2 suspension with it before. The yield was still quite high, as you've seen.
What was the purpose of the gelatin, after all?
I think the catalytic action of a hundreth ppm of metal ions is only true for the un- gelatinized reaction mix.
Metal ions still have to be avoided, but the reaction isn't hyper- sensitive. | Quote: |
The gelatine is used as a chelation agent to tie up metal ions , mainly copper is the worst for poisoning the hydrazine reaction . The metal ion data
was in reference to the stability of the hypochlorite solution , where any impurity
is catalytic in the decomposition of the hypochlorite . But that won't be a problem for you since you won't be storing the solution .
However it will
be a factor in the hydrazine synthesis
since what makes bleach unstable also
hinders the formation of the hydrazine .
| Quote: |
To make my 10% NaOCl, I will have to chlorinate the NaOH until saturation, and then quickly add some extra NaOH (until the chlorine smell disappears)
to stabilize the NaOCl. I hope this works. |
Keep it really cold towards the end of what you are planning to do . You are working contrary to the physical chemistry
which applies .
| Quote: |
To produce chlorine from TCCA, I simply add dilute (15- 20%) HCl. The reaction is smooth and steady (it can be controlled by warming or cooling,
warming is usually necessary). Not only the chlorine of the TCCA, but also the chlorine of the HCl is converted to Cl2 gas, making this a very
effective method. |
That's an interesting method for chlorine .
TCCA has not received much attention in regards to its usefulness in synthesis .
That's a good one to know . |
|
|
|
|
redneck
Harmless

Posts: 12
Registered: 26-9-2004
Member Is Offline
Mood: No Mood
|
|
Hi guys
What recipe are you using? Where can I get it? Who is Mr A. , and where can I get his recipe?
@ Rosco Bodine
What is a mid file? I opened it with Winamp but I didn´t hear anything.
I don´t believe sodium hypochlorite is made the way you descibed on an industrial scale. I think it would be cheaper to make it via electrolysis of
an cold aquaeous solution of sodium chloride.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by redneck
Hi guys
What recipe are you using? Where can I get it? |
http://www.sciencemadness.org/talk/viewthread.php?tid=757&*
| Quote: |
Who is Mr A. , and where can I get his recipe? |
Speak of the devil , ........
and then look above 
| Quote: |
@ Rosco Bodine
What is a mid file? I opened it with Winamp but I didn´t hear anything. |
Try Winblows media player and enable midi as a default file type association .
| Quote: |
I don´t believe sodium hypochlorite is made the way you descibed on an industrial scale. I think it would be cheaper to make it via electrolysis of
an cold aquaeous solution of sodium chloride. |
Actually the same plant usually produces three products , sodium hydroxide , chlorine , and sodium hypochlorite .
It allows for continuous operation , and diverting production into what is sold separately or already combined .
|
|
|
redneck
Harmless

Posts: 12
Registered: 26-9-2004
Member Is Offline
Mood: No Mood
|
|
@ Rosco Bodine
Thank you for the link!
Strange music. Sounds like south american indian style. I prefer Carl Cox when I´m working. He helps against tiredness.
I tried to make hydrazine sulphate from ammonia. I read recipes in the internet which use 2.5 to 25 times more ammonia than stochiometrically needed.
But I didn´t want to have a terrible smell smell in the garage again. So I used not more ammonia than theoretically needed.
@ garage chemist
The chlorox I have is labelled as containing 2.8 % NaOCl. But when I boiled it down untill dryness I received 13 % of an unknown salt. If it was
NaOCl*6 H2O the chlorox would contain 5.7 % NaOCl.
I also tested how much chlorine gas is generated when I add HCl 30 % to the chlorox. From the amount of gas I calculated the amount of NaOCl in the
chlorox. My result was 6 %.
So I figured out that the label was wrong. The chlorox contains not 2.8 % NaOCl but 2.8% Cl.
So the chlorox contains 5.88 % NaOCl for real.
My hydrazine sulphate experiment
2 NH3 + NaOCl ==> N2H4 + NaCl + H2O
I poured 20 ml chlorox into a test tube. Then I dissolved 13 mg gelatine and 36 mg sodium acetate (It´s also a heavy metall ion catcher) in it. Then
I cooled it in a water bath and slowly added 2.2 ml 25 % ammonia. The tube would become warm by the reaction heat and the ammonia would evaporate
without cooling. During the addition of the ammonia there was some foaming and after the addition a tiny but steady stream of bubbles coming out of
the mixture. Then I added 36 mg dirty urea and boiled the solution down to one third of it´s original volume.
Then I let it cool and afterwards I cooled it in a water bath and added 3.2 ml 38 % sulfuric acid in small portions because a lot of gas was generated
and it foamed. When half of the acid was added there were tiny crystals in the solution. I filtered them of and added the rest of the acid to the
solution but no more crystals were formed. But the next day there were large crystals in the size of one inch long needles in the solution. But there
were also medium size cubic crystalls in the solution which look like table salt.
Now the question is are these needles hydrazine sulfate?
Are the cubic crystalls NaCl or are they also hydrazine sulfate?
How can I find out what they are?
What are the tiny crystals that formed immediatly when I added the sulfuric acid?Can I use more gelatine without turning the solution into a pudding
like mass?
(How many per cent compared to the boiled down solution)
Could anybody post how hydrazine sulphate crystals look, please. What is their shape?
[Edited on 29-4-2005 by redneck]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Boiling NaOCl breaks it down into ClO3 + 'Cl.
Doesn't adding ammonia to bleach release chlorine instead? Or am I thinking of acid?
Tim
|
|
|
redneck
Harmless

Posts: 12
Registered: 26-9-2004
Member Is Offline
Mood: No Mood
|
|
3 NaOCl ==H+, >70°C==> NaClo3 + 2 NaCl
NaOCl converts to NaClO3 only if acidified. In an alkaline solution it´s stabe.
NaOCl forms chlorine gas when it reacts with HCl not with ammonia.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Ammonia and bleach give chloramine (Cl-NH<sub>2</sub> , Tim. , Tim.
Rosco, why don't you just tell everybody else who Mr. Anonymous is?  
Somewhat related to the topic: how long will stored hydrazoic acid last before decomposition?
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by sparkgap
Rosco, why don't you just tell everybody else who Mr. Anonymous is?   |
Mr. Anonymous and Rosco Bodine are aliases . These aliases conceal
the true identity of Rosco P. Coaltrain , sheriff of Hazzard County ,
to keep him out of trouble with Boss Hogg , chief of animal farmland security 
| Quote: |
Somewhat related to the topic: how long will stored hydrazoic acid last before decomposition?
sparky (~_~) |
The dilute solution is reportedly stable in the absence of light and kept cool .
But such solutions are an extreme danger due to the toxicity and volatility .
The material is so toxic that it has been said if you smell it , then you have
already been poisoned . And that is not very much if any exaggeration .
Extreme Caution is the imperative rule for hydrazoic acid . It simply is not
the sort of material inclined to suffer fools or carelessness without exacting
a dear price for the error . It is among the deadliest toxins known to science .
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
The container of hydrazoic acid I saw in our lab was being kept at quite cool temperatures, so I guess its volatility and rate of decomposition should
be reduced. Still, I'm thankful I've never needed to use it in any of my experiments.
I thought as much about the toxicity.  IIRC, azide ion is a good inhibitor of the
ETC so crucially needed in respiration. IIRC, azide ion is a good inhibitor of the
ETC so crucially needed in respiration.
Thanks, Mr. Anonymous. 
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The recent discussion about chlorination of NaOH has caused me to remember that this came up once before , and I will have to check my patents , but
IIRC there is a more efficient method for use of chlorination in the synthesis of hydrazine and it involves chlorination of a slurry of urea to form
chlorourea , which is then
reacted with a base like sodium carbonate
or sodium hydroxide to form hydrazine upon heating and hydrolysis . IIRC , there is no need to even make sodium hypochlorite , if you are going to go
the chlorination route , and the yield is better by the chlorourea intermediate . I will see if I can find the patents which reference the method .
GB1063893 Hydrazine or Semicarbazide selectively produced from monochlorourea
Related semicarbazide patents are
US5241117
GB1153483
[Edited on 4-5-2005 by Rosco Bodine]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Wow, that chlorourea route would be great if it worked! Thanks for the suggestion.
It reminds me of a method to produce methylamine: first acetamide is mixed with bromine, then the formed N-bromoacetamide is treated with a base to
form methylamine.
That should work with urea, too!
I have about 7ml of bromine, so I could try it out.
A method for determining if a precipitate which occurs on adding H2SO4 is indeed HS, one could dissolve it in some ammoniacal AgNO3 solution. HS
immediately produces a silver mirror, without any warming.
[Edited on 4-5-2005 by garage chemist]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Earlier in the thread a couple of mentions were made of the urea - hypochlorite method having been performed successfully using lower concentration
bleach . I have never tried the use of the lower concentration simply because I was seeking to obtain the greatest amount of product per liter of
reaction mixture which would be processed . But there is a German patent which refers to what is probably the invention of this
" Schestakow reaction " for hydrazine from urea and hypochlorite , and the original patent describes dilute hypochlorite of 3% being used ,
if I have understood the German text correctly .
See attached file .
Anyway , the conditions under which hydrazine forms and can be isolated from such reactions are very specific . In different molar proportions and
conditions of pH and temperature and reaction time ,
indeed urea and hypochlorite mutually decompose each other without the formation of hydrazine . And this fact makes urea a patented decontaminant
used for the environmentally safe destruction of waste hypochlorite solutions . So from this it is known that the manufacture of hydrazine is a very
specific and controlled process for a narrow range of conditions . Departure
from that " window " reaction condition results in yields of hydrazine rapidly deteriorating to zero . This fact is why I
tried to emphasize earlier that changing anything about the " optimized " method would reduce the yields . For information
related to the conditions for urea being used for the destruction of hypochlorite see US4508697 , US2784057 .
An off topic digression here concerning urea and a possible reaction product with sulfur , hydrogen sulfide , or sodium hydrosulfide / sodium
polysulfide . I cannot recall for certain where I read a passing reference concerning such a reaction , or even if it is perhaps just something in
the way of a contemplated experiment which occurred to me out of the blue . It was an idea in regards to producing a fusible and or soluble
organo-sulfur compound which may have usefulness as a fuel in propellants , or as a fuel/sensitizer for AN compositions , or as a possible sulfide
type reducing agent .
I have searched exhaustively and can find no reference to such a urea reaction . If anyone knows of something like this or
a reference , please refresh my memory .
[Edited on 4-5-2005 by Rosco Bodine]
Attachment: DE164755 Schestakow , Hydrazine by Urea Hypochlorite.pdf (157kB)
This file has been downloaded 2034 times
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Thanks a ton for the german patent. Too bad I can't read it at the moment... my computer crashes as soon as it has fully loaded. That's my
old computer... my better one is being repaired at the moment.
Anyway, today I have sucessfully produced sodium azide.
I did the freebasing with ethanol since I was running low on IPA and ethanol is just easier to get.
To 13g HS, I added 18ml denatured ethanol (94% Vol.). Then 4g of NaOH were added.
Upon stirring with a glass rod, the salt mixture became dough- like and eventually turned into a homogenous sticky white mass. The mixture was quite
hot. I wanted to proceed quickly in order to not lose too much hydrazine due to air oxidation.
I added another 5g NaOH, and suddenly the mixture started boiling violently. White fumes were produced, and some of the solution was spilled. I
quickly added a dash of ethanol to cool it. This stopped the boiling for a moment, but it started boiling again as I kneaded the mass with the
stirring rod.
I had to add even more ethanol.
But finally, only a dry, very fine and rather voluminous precipitate of sodium sulfate remained under the ethanol.
The solution was decanted and the sodium sulfate washed three times with 10ml ethanol each time (the precipitate sucked up a lot of ethanol and was
rather voluminous).
The combined solutions were stored in a tightly closed round bottom flask.
5,5g NaOH were dissolved in 60ml ethanol (took quite a lot of time). This was then mixed with the hydrazine extract. It was nearly 100ml of liquid.
18,7g IPN were prepared from 15g NaNO2, 17g IPA and 40ml 25% HCl (an excess of IPA was used in order to obtain a stable IPN). The NaNO2 was dissolved
in a minimal amount of water and the IPA was added. Then, while cooling in an ice bath, the HCL was slowly added. The IPN yield was very good (over
90%). It was washed with bicarbonate solution and "dried" for 15 minutes with CaCl2 (I could have omitted that step, it was not necessary).
The ethanolic NaOH/hydrazine solution was placed in an ice bath and one third of the IPN was added. After 15minutes of stirring, there was no evidence
of any reaction. The second third was added. Again no reaction. The last third was added, again without any signs of precipitate forming. I took the
solution out of the ice bath and waited for it to warm up. Finally, after 30min, it became more cloudy and an extremely fine precipitate began
forming. It became a bit warm and I stuck it into the ice bath again.
The amount of precipitate slowly increased. I am letting it stand overnight in order to complete the reaction. The precipitate looks
"milky", it is really very fine.
I observed the following things:
- The freebasing is VERY exothermic! After adding the first portion of the NaOH, the solution MUST return to room temperature before adding the second
portion. The container should be covered during the cooling.
And 18ml of ethanol is not enough. 25ml would more reasonable.
- The diazotation reaction took an awful lot of time to start and then only proceeded lazily. I think this was due to the rather dilute solutions I
used. I should have used less ethanol to dissolve the NaOH. And I should give the sodium sulfate from the freebasing more time to settle so I can use
less ethanol for the washings.
That's how one learns! My next azide batch will be much better and without incidents like the hydrazine extract starting to boil.
[Edited on 4-5-2005 by garage chemist]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
When I do the freebasing which will later be extracted with methanol , the process
of reacting the solids first to form an aqueous phase is done before any alcohol is added . This allows for the exotherm to be managed , and
actually it is essential to maintain
that exotherm at nearly the boiling point to keep the slurry liquid . The slurry is only allowed to cool just to the boiling point of the alcohol so
it won't flash boil the alcohol when it is added . The first alcohol extract is still very warm when it is decanted . This works fine for
methanol but not for isopropanol . I am unsure about ethanol
but I seem to recall that it worked okay and didn't phase separate like isopropanol .
Read my earlier post about the alternating additions which really helps the solubility for the freebasing .
You really need to be doing the freebasing in an Erlenmeyer with a stirbar on a magnetic stirrer . A glass stoppered
flask is best . The NaOH should be preweighed and quickly placed into a small plastic bottle having a capped conical dispenser tip , like a mustard
bottle . The stirbar should be set at slow speed and about half the HS put into the flask . To the stirred dry mixture can be added in portions the
NaOH , stoppering the flask and capping the NaOH bottle between additions . If you follow this method , you should get good results .
A drop or two of water may be required to kickstart the reaction which then produces its own water as it proceeds ,
melting the solids in the byproduct water
which self heats as it goes . What you are actually getting as the liquid , is hydrazine dihydrate , plus any water you may have to add , the less the
better of course .
[Edited on 4-5-2005 by Rosco Bodine]
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Just a writeup
12 grams of sodium hydroxide was dissolved in 35 ml distilled water and added with the aid of a sepretory funnel (tight fitting ground glass) with
magnetic stirring to 20 grams of hydrazine sulfate. The additions were done over the course of and hour and a half during which very slight heating
was observed.
At no point did everything become liquid but I assumed the heavy precipitate at the bottom was sodium sulfate that had been produced. This mixture as
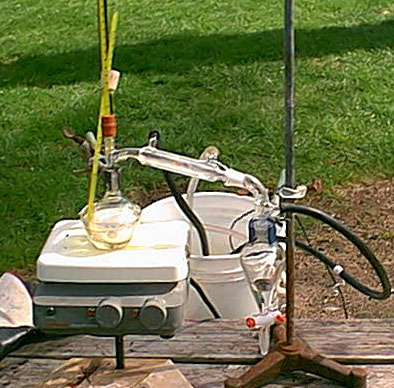
then set up to distill. The apparatus is in the attached picture. I distilled into a sepretory funnel so that I could take off the distillate at
different times. The gasses were simply lead away and bubbled into water.
The distillate started to come over when the water bath was at about 130 C and the still head only rose to 100 C so it was mostly water coming over.
The bath temperature continued to climb until it held at 152 C and the stillhead continued to hold at 100 C. A portion of the liquid distilling was
removed and a few drops were added to a test tube of bromine water, immediately removing the color. So a positive test for reducing character. A
large portion of the hydrazine came over in this region, too much water, too afraid of distilling anything less then the azeotrope (hydrazine hydrate)
at 120 C. It took a lone time to remove the water, the amount of azeotrope expected to come over was only 6.75 ml and therefore it came as no suprise
when after 17 ml of water came over I only had a short break around the point of the azeotrope before there was a temperature drop and liquid stopped
distiling. Additionally the flask was bumping quite a bit and I didn't want to press it considering it was hydrazine.
I know there are better ways to get the hydrate but this way has always bekoned to me, now I only have 480 g hydrazine sulfate left....
BTW, does anyone have any details on the preparation of anhydrous hydrazine by distilling it from hydrazine borate? Expecially the preparation of
said compound?
[Edited on 5/6/2005 by BromicAcid]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I have a reference but only the obvious directions.
"Reaction of the hydrate with boric acid gives hydrazinium borate which can be dehydrated by heat and decomposed to N2H4 and B2O3 by furthur
heating."
And the reference quoted with it is Gmelins Handbuch der anorganische Chemie, Berlin, Verlag Chemie, 1928. 8th ed., System 21: p313-314.
I'd be interested to read that myself.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Cyanuric acid similarly forms a salt with hydrazine which decomposes at high temperature to the free cyanuric aicd and free hydrazine . But the
decomposition temperature is not stated , except as being below 360 C , which is damned hot .
Any of the distillation schemes for anhydrous hydrazine , even for the hydrate actually , are going to result in decomposition losses . The ketazines
may be thermally unstable , and this is a possible route . I know the ketazines are
hydrolyzed to the salt by acids , and perhaps they may also be hydrolyzed by
gaseous ammonia , which could be another possible route for the hydrazine monohydrate . The hydrate can be dehydrated by highly activated and dry
alumina , but the adsorption is very small before the alumina has to be high fired again to get it dry enough for recycling .
It's only good for something like 3% of the weight of the alumina in absorbed water , till the deydrating property is lost .
Trying to get the water out of hydrazine
is something like trying to get the water out of sulfuric or phosphoric acid . It is
extremely difficult , and that is precisely why it is best to work with the freebasing method . Obtaining the hydrazine in a highly concentrated and
dehydrated form
is a process plagued by losses and difficulty which should be avoided for any scenario where the hydrazine is not required for synthesis to be used in
such concentrated form .
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I think anyone desperate enough to try that with ketazines would be better off doing ammonolysis directly on hydrazine sulphate with liquid ammonia or
by using an entraining method on hydrazine hydrate. I agree with you though, anhydrous hydrazine is seldom useful and rather hard won.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I was able to open the PDF by first saving it and then opening from there.
The Schestakow process actually uses 3% NaOCl on urea, but the hydrazine is isolated by addition of benzaldehyde to the reaction mixture, benzaldazine
is formed and this is seperated and hydrolysed to benzaldehyde and hydrazine sulfate with sulfuric acid.
I am unsure if the solubility of hydrazine sulfate is low enough to allow it to crystallize out from the dilute solution obtained by using 3% NaOCl.
Does anyone know the solubility of HS at 0°C? The solubility is always listed for 25°C.
EDIT: I will also try out the chlorourea process.
[Edited on 6-5-2005 by garage chemist]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
System 21 of Gmelin's is sodium. The original article that Gmelin's is supposed to point to may be J. Russ. Phys. Chem. Soc. 34, 227 (1902),
by Djavachoff, and the temp is over 260C to form hydrazine in that article.
As for Roscoe's complaint in the azide thread about the move (of discussion of preparation of hydrazine from hypochlorite and urea or whatever)
from the azide thread to this one - I should comment since I'm the one who wondered how many more pages of azide thread the preparation of
hydrazine was going to take. It seemed obvious to me that two full pages of this with no end in sight (see DPPP thread) was out of place, given that
there was already this thread, and it would certainly be of more use here.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by S.C. Wack
As for Roscoe's complaint in the azide thread about the move (of discussion of preparation of hydrazine from hypochlorite and urea or whatever)
from the azide thread to this one - I should comment since I'm the one who wondered how many more pages of azide thread the preparation of
hydrazine was going to take. It seemed obvious to me that two full pages of this with no end in sight (see DPPP thread) was out of place, given that
there was already this thread, and it would certainly be of more use here. |
Actually , the matter of the off topic nature of the preparation of hydrazine sulfate
had already been noticed , and mentioned before you posted the question
about how many more hydrazine posts in an azide thread would there be . And I fully agree that the preparation of hydrazine sulfate is off topic in
the azide thread . Splitting the thread and exporting the synthesis of hydrazine sulfate posts here was okay by me .
No problem there .
But a half dozen posts that were azide specific were mixed in with the posts which were exported here from the other thread . What I meant to say is
it was more than just the offending
off topic posts which got moved . I shouldn't have said anything because I bet I just volunteered to be the one to sort through those posts and
pick out the candidates for
" reintegration " .
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Hmm, it's a tricky one since things are so interlinked - the production of Hydrazine and azide - particularly when a single post contains
descriptions of both (something I cant split easily without disrupting the flow).
If there's a specific post that I overlooked and doesn't belong here, let me know.
Suggestions welcome. I would only want to perfect the layout of such a high-level discussion 
I will remove this post later, once/if I got suggestions (U2U pls).
PS oh yeah rosco you are damn right 
[Edited on 7-5-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I did not have good sucess with the alcohol- free freebasing.
I mixed 13 HS with 4g NaOH, it got warm and after 2 hours with occasional stirring the mix slowly liquefied.
But on addition of the next 5g NaOH, nothing happened, the mix cooled down and the NaOH showed absolutely no signs of reaction.
I extracted it with ethanol three times and there were still unreacted portions (clumps) of the powder mix, even though I stirred it a lot.
With the addition of alcohol to the HS at the beginning (my previous method), I got a complete reaction and a fine precipitate of sodium sulfate.
If it can be cooled efficiently, this method would be optimal.
[Edited on 11-5-2005 by garage chemist]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That's strange . I suspect your hydrazine sulfate is not pure ,
maybe a lot of salt or sodium sulfate as an impurity .
The freebasing generally is a very energetic and exothermic process and
proceeds rapidly . Five minutes , not two hours for a tenth mole of HS
would be more like it . And the exotherm proceeds
right to the end or almost the end . You really have little to no free hydrazine appearing until half of the NaOH has been added , since all you are
doing is converting the hydrazine sulfate to dihydrazine sulfate to that point , if there is good mixing . So you can add the first half of the NaOH
as rapidly as the exotherm allows and in fact the idea
is to get the mixture very hot , so everything dissolves as a hot concentrated solution of dihydrazine sulfate . Only during the additions of the
remaining NaOH does the actual hydrazine freebasing occur , simultaneously with the precipitation of
anhydrous sodium sulfate forming a slurry
in a hot liquid phase which is decreasingly
dihydrazine sulfate , and increasingly converted to hydrazine dihydrate
by the time the final amount of NaOH is added . IIRC the freebasing of 2 moles of HS , took perhaps a half hour to the point where the first of the
alcohol ( methanol )
was added . And a smaller mass like a
tenth mole would likely require supplemental heating , not cooling ,
in order to maintain everything in solution
at the midpoint of the reaction , before the actual freebasing part of the reaction begins with the addition of the remaining NaOH . You can follow
the course of the reaction by observing the the exotherm and the solubility changes which are very predictable .
|
|
|
| Pages:
1
..
3
4
5
6
7
..
24 |