Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Under what conditions do alkyl azides rearrange to imines?
Under what conditions do alkyl azides rearrange to imines? I am having some difficulty finding details on this one. I know that reactions involving
free HN3 are unsavory, but I was unaware of its reaction with alkenes, and was also wondering if it can be radicalized to affect to affect
anti-Markovnikov addition using light or peroxide, similar to how addition with HX is affected.
http://organicreactions.org/index.php?title=Schmidt_reaction...
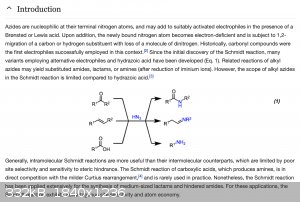
https://www.organic-chemistry.org/namedreactions/schmidt-rea...

"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Nakhimov
Harmless

Posts: 16
Registered: 8-12-2018
Member Is Offline
|
|
Here is a recent paper with some in-situ reactions of alkyl azides in the presence of copper catalysts: https://www.thieme.de/statics/bilder/thieme/final/de/bilder/...
Additionally, here's a proposed mechanism from the Hu paper:
http://advances.sciencemag.org/content/advances/3/8/e1700826...
(doi:10.1126/sciadv.1700826)
[Edited on 12-18-2018 by Nakhimov]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Here is a pretty interesting overview of the Schmidt reaction.
Attachment: wrobleski2012.pdf (2.9MB)
This file has been downloaded 378 times
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
What bothers me about this is how weak of an acid HN3 is. How would it easily add to an alkene?
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|