UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
concentrating nitric acid
I finally managed to try to concentrate the nitric acid I made in my Birkeland Eyde reactor. I started with 500mL of 1.25 molar nitric acid (measured
via titration), heated it on the stove until less than 42mL were left, which theoretically should result in the maximum 68% acid by weight azeotrope,
assuming only water is driven off:
Since this was my 1st attempt and I wanted to see how high a concentration I can get, I let it evaporate until ~25mL was left. However, titration
indicated it was only 40% HNO3 by weight.
Is there any tricks to boil the water off faster than acid, or will I have to boil off significantly more to achieve 68%?
Also, can 40% nitric acid be used for most recipes that state to use 70% acid, namely for nitrocellulose, Pb(NO3)2, and RDX? If it still works, then
I'm satisfied with 40%.
|
|
|
jarynth
Hazard to Self
 
Posts: 76
Registered: 12-8-2008
Member Is Offline
Mood: No Mood
|
|
Congratulations on your results, which shed new hope on amateur industrial chemistry! Could you post some pictures of your setup?
The acid is more volatile than the water; it also tends to decompose. Instead, I would try to make sodium nitrate with the dilute acid. Crystallize it
then mix it with conc. sulfuric acid to get conc. nitric acid by distillation. Save the NaHSO4, which on heating gives back H2O, H2SO4 and SO3 (I saw
the procedure here somewhere) and the base, which can be recycled.
40% HNO3 cannot be used for most nitrations. You could use it for making mononitrotoluene, maybe, but not much else. Some HNO2 present in solution
might be affecting your titration and has to be removed by treatment with urea (I don't remember if you already posted that you did this step).
Otherwise I cannot think of other major impurities that could taint your product (maybe some metals from the electrodes). If you manage to get a
precise measurement of the concentration, your product will be suitable for many 'milder' reactions (analytics, etc).
[Edited on 19-10-2008 by jarynth]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Read up on how fractional distillation works. When you are evaporating a mix of two liquids, regardless if there is an azeotrope or not, some of the
higher boiling component boils off along with the lower boiling on; it's not a sharp 'all of A boils off until only pure B is left' situation. This
hold even for H2SO4, once you get past around 75% H2SO4 noticeable amounts of acid start coming over with the water.
So you really should be using some sort of fractionation once you get above 10% or so. You could concentrate to 25% to 30% in a simple distilling
rig, and recover the acid that distills over during that step, then finish off in a fractionating system. Somewhat reduced pressure during
concentration could help reduce decomposition, as you want reflux during fractionation you'd need a condensing still head for that step.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
If you use subconcentrated HNO3 in cellulose nitrations, you'll get lower nitrogen containing NC (pyrocellulose).
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
jarynth, I've posted my results on the main Birkeland Eyde reactor thread.
Back to about using 40% HNO3. If I understand correctly, I think 40% nitric acid should be sufficient to make gun cotton as long as there's enough
sulfuric acid to absorb the water. I think nitration stops when water accumulates, so the sulfuric acid is used to dehydrate it, as a H2SO4 + sugar
=> huge black turd experiment suggests. I'm not sure if this applies for RDX or other explosives.
[Edited on 20-10-2008 by UncleJoe1985]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
It's correct that it works to dehydrate, but the acid itself is part of the nitration system. I can't say if an excess of H2SO4 will yield the high
nitrogen guncotton, though have some doubts since it may dilute the nitronium. Simple theoretical substitution will not always necessarily work since
the material can react differently at different concentrations. Hexamine and dilute HNO3 under cooling yields hexamine mononitrate, with conc. HNO3
the result is hexamine dinitrate.
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Simple theoretical substitution will not always necessarily work |
Well, I'm not too concerned with making nitrocellulose and high explosives. My first priority is making Pb(NO3)2 for PbO2 anodes.
One thing that I didn't mention was that when boiling the mixture, there were thick, white, nauseating fumes. Even after I cooled and bottled it,
faint white fumes were still seen. Does this mean the acid is more concentrated than I calculated?
[Edited on 20-10-2008 by UncleJoe1985]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
That was most likely the nitric acid evaporating with the water - which is about all you can expect without properly distilling it to concentrate. 40%
is plenty strong for making lead nitrate - from lead sheet, litharge, carbonate, red lead or whatever you like. I usually mix mine up about 20-40% for
dissolving lead. If it's too strong you tend to get a lot more fumes, NO2 and evaporation. I distill my HNO3 from nitrate salts, ends up 96-99%
depending on how good the batch of drain cleaner was.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
The trick would be, theoretically (I didn't try it personally), to let the dilute HNO3 react with BaCO3, OR CaCO3, OR maybe even PbCO3. All those 3
reactions would give a nitrate, that should react with H2SO4 to give insoluble Sulfate (BaSO4, CaSO4, PbSO4), and HNO3 !
Since the H2SO4 can be concentrated by heating, this would be the way to obtain even 99 % HNO3 !
(theoretically ...)
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
Thanks for the tips, but I'm trying to do this with minimal additional chemicals. The NaNO3 + H2SO4 jarynth might be promising, depending on how easy
it is to reclaim the sulfuric acid.
I'm planning to try fractional distillation (without H2SO4 to prevent water from evaporating) by boiling the aqueous solution and collecting the fumes
where the temperature is ~80 C. Since the water, nitric acid azeotrope has a boiling point of 120 C, I'm afraid that I won't be able to collect much
acid if the vapor also condenses at 120 C. Is this the case or will most of the HNO3 vapor separate from water vapor and condense at ~80 C?
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Save the NaHSO4, which on heating gives back H2O, H2SO4 and SO3 (I saw the procedure here somewhere) and the base, which can be recycled.
|
2NaHSO4 = Na2S2O7 + H2O
Na2S2O7 = Na2SO4 + SO3
The second reaction doesn't happen very easily, and Na2SO4, the product that isn't really a base, doesn't decompose easily to a base. It could be
reduced to sulfide, but its all really a waist. worrying about recycling the bisulfate is likely to hard, just re-crystallize it and use it for other
things if you must. It has its own uses in the lab.
| Quote: | Since the H2SO4 can be concentrated by heating, this would be the way to obtain even 99 % HNO3 !
(theoretically ...) |
| Quote: | | I distill my HNO3 from nitrate salts, ends up 96-99% depending on how good the batch of drain cleaner was. |
The azeotrope(sp) with sulfuric acid and water is something around 98%. Your not going to get 99% Nitric acid especially if there are salts dissolved
it it and your not using a vacuum. A significant amount of the would likely decompose into NOx.
Purifying the Nitric acid from the dilute solution seems like a problem. It seems a significant amount of it evaporates while the solution is
concentrating. Perhaps if you monitored the temperature at which it boiled at, you could stop when it became reasonably concentrated which you'd know
since different concentrations of the acid boil at different temperatures, then distill it with concentrated sulfuric acid to get the remaining water.
You could then heat the sulfuric acid to dehydrate it and re-use it to dry/distill more nitric acid.
I bet this would probably increase the yield of Nitric acid instead of just boiling it away.
[Edited on 25-10-2008 by kclo4]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by UncleJoe1985...
I'm planning to try fractional distillation (without H2SO4 to prevent water from evaporating) by boiling the aqueous solution and collecting the fumes
where the temperature is ~80 C. Since the water, nitric acid azeotrope has a boiling point of 120 C, I'm afraid that I won't be able to collect much
acid if the vapor also condenses at 120 C. Is this the case or will most of the HNO3 vapor separate from water vapor and condense at ~80 C?
|
Starting with a dilute solution, what will distill is first water, then water with increasing amounts of HNO3 at a slowly increasing temperature until
the azeotrope is hit. If there was an excess of nitric acid, once all the water had been distilled out the temperature would drop and the distillate
would containing higher and higher concentrations of HNO3 unril pure acid was distilling; as you are talking about dilute solutions this part of the
sequence will never happen. If the starting acid is much less concentrated than the azeotrope, you'll never reach and distill the azeotropic mix as
the HNO3 will come over with the water as a more dilute mix.
To concentrate the acid to the azeotrope, you will want to fractionally distill the dilute acid. Doing so will result in the vapors at the top of the
column staying near 100C and being water or very dilute acid, then quickly rise to the azeotrope point of 120.5 (with delta from actual atmospheric
pressure) and staying there. Don't try to distill dry, leave a bit of liquid especially if there are dissolved salts; those tend to cause bumping
BTW.
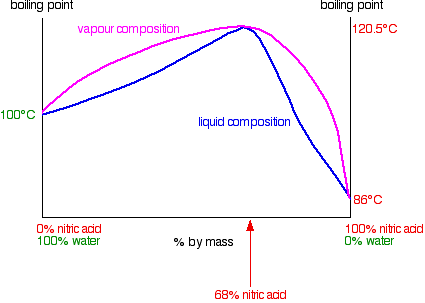
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
Actually, I've seen that diagram before, but wasn't sure what the vapor composition curve meant. I've figured it out after going back to the lecture.
If my understanding is correct, water is the more volatile component compared to the azeotrope, so by boiling the solution, you get a higher
composition of water in the vapor, which gets even higher as it condenses and is vaporized again by the vapor below. That means, all I have to do is
to put another bowl with a hole in it over my previous glass bowl and make sure the temperature at the hole is 100 C. I can't believe I wasted so much
acid not realizing such a simple procedure.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
What material would you use for the HNO3 distillation apparatus if you wanted to concentrate HNO3 on a fairly large scale, i.e. other than a
laboratory-scale glass distillation flask and condenser? Other than glass, there is not much to choose from. Plastics such as PVC are ruled out by
the temperatures required. Perry section 23 indicates that the only common fabrication metals that are entirely satisfactory for handling the stuff at
the temperatures and concentrations required are zirconium and 14% Si steel; while austenitic 18%Cr-8%Ni and type 316 stainless steel are satisfactory
for handling it only at temperatures below the boiling-point.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
Distilling is much more trouble than the salt reaction:
Ba(NO3)2 + H2SO4 ==> HNO3 + BaSO4 ; the BaSO4 can just be filtered off ! (theoretically).
Of course, this needs H2SO4 ... That's why I mentioned the Pb-version of the reaction:
Pb(NO3)2 + H2SO4 ==> HNO3 + PbSO4 ; PbSO4 is as insoluble (in water) as BaSO4.
But the PbSO4 might be thermically be decomposed (??) or not ?
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
JohnWW, How much do you plan to distill? I bet a large RBF would do, they do after all go up to ten to twenty liters. Or perhaps you could try
something like having a L liter flask, and then an addition funnel to constantly add the nitric acid to distill it?
That, or you could perhaps Find metal coated in Teflon? Perhaps that could hold up? I kind of doubt it.
Cheif, Distillation isn't even that bad. Messing around with toxic compounds such as lead might be however since they can cause a lot of problems.
For concentrating Nitric acid, Would it be possible to freeze out some of the water or the acid? It can be done with other compounds (i.e H2O2) so
maybe it can be done with Nitric acid.
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
I got the idea of using PTFE (teflon) coated metal vessels for distilling. I was using a glass bowl earlier, which is a poor heat conductor and can
potentially fracture from heat stress.
Does any body know how to coat cookware with enough teflon so that it can be used for HNO3 distillation? I saw various sprays, but don't know what's
really in them. I don't feel like finding out myself - metal + HNO3 => thick, poisonous NO2 fumes. Does anyone know if it's safe?
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
| Quote: | Originally posted by chief
Distilling is much more trouble than the salt reaction:
Ba(NO3)2 + H2SO4 ==> HNO3 + BaSO4 ; the BaSO4 can just be filtered off ! (theoretically).
Of course, this needs H2SO4 ... That's why I mentioned the Pb-version of the reaction:
Pb(NO3)2 + H2SO4 ==> HNO3 + PbSO4 ; PbSO4 is as insoluble (in water) as BaSO4.
But the PbSO4 might be thermically be decomposed (??) or not ? |
Lead sujlfate is soluble in alkali hydroxides. You dissolve in sodium hydroxide to get sodium sulfate and sodium plumbate, then add hypochlorite to
deposit lead dioxide. Reduce dioxide with hydrogen peroxide and you have lead monoxide or litharge. But then again you might have to use acid for
the peroxide to reduce I have not tried this without acid.
Fellow molecular manipulator
|
|
|
renatomerino
Harmless

Posts: 2
Registered: 19-8-2008
Member Is Offline
Mood: No Mood
|
|
If the nitric acid has traces of hydrochloric acid can be set using silver nitrate.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by UncleJoe1985
I got the idea of using PTFE (teflon) coated metal vessels for distilling. I was using a glass bowl earlier, which is a poor heat conductor and can
potentially fracture from heat stress.
Does any body know how to coat cookware with enough teflon so that it can be used for HNO3 distillation? I saw various sprays, but don't know what's
really in them. I don't feel like finding out myself - metal + HNO3 => thick, poisonous NO2 fumes. Does anyone know if it's safe?
|
I haven't tried teflon. For distilling the liquid it's worth the investement for a distillation flask or better glass retort. For stoppers, I have
rubber stoppers I've wrapped in Al foil and used that to distill fuming HNO3. The foil gets only slightly to barely attacked. Better would have been a
glass stopper.
|
|
|
renatomerino
Harmless

Posts: 2
Registered: 19-8-2008
Member Is Offline
Mood: No Mood
|
|
The use of glass no danger of fracture in warm environments and constant temperature.
In the concentration of nitric acid is used column of dishes.
|
|
|
Texium
|
Thread Moved
19-11-2023 at 12:43 |