Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
Chromatography materials
- If I make silica by adding HCl to waterglass, and then roasting and grinding the silica, will it be OK for TLC when glued to a glass slide? Or will
it not have a high enough surface area compared to the 'real thing'?
- If a plain aluminium plate is soaked overnight in dilute acid, it will form a layer of Al2Cl3 which then converts to alumina (right?). Would this do
for a plate, or would it suck? I might try it anyway but I don't have any real plates for comparison.
- If anyone has experience making their own plates let me know. I suspect it's more difficult than the procedures outlined above.
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
The home-silica is fine for TLC purposes, it use dto made this way even a decade ago in some schools. The problem is having reproducible results, as
only minor changes during the preparation ahve major influences on the specific surface and density, etc.
I would recommend letting it gel nicely after reaching neutralization, easily 30-120min. Filtering can be a pain, and you need to wash the silica very
well to remove the NaCl and eventual impurities from the acid.
I have used such home-made silica on several occasiosn for severalpurposes, but not TLC. Column chromatography (tricky, I prefered buying commercial
silicagel), and catalyst support.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
If you are interested in making your own TLC plates, you might want to check out this website.
PS Does anyone know a good source in the US for TLC plates? Why are they so expensive?
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
Thanks for the information guys! Good point about reproducability, Klute. I will mostly be using TLC to compare feedstock spots to product spots, so
if the silica for both plates was produced in the same batch I imagine that would suffice for a simple comparison.
Smuv, thanks for the link. Sounds like a drop or two of PVA is the way to go..
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Have you tried paper chromatography?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Maja
Hazard to Others
  
Posts: 143
Registered: 27-2-2006
Member Is Offline
Mood: No Mood
|
|
Maybe someone can explain how the colored spots can appear on the plate ? I can't understand how TLC plates works...
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, eithe ryou sue untreated plates and spray the eluted plates with a revelant, ei soemthign that will color any organics laying around
(H2SO4/vanillin, ninhydride for aminoacids, KMnO4, etc), or expose the plate to vapors (I2).
The bets is to use a additive that is fluorescent under UV, zinc silicate IIRC, so that when you expose a fresh plate to UV all the surface is
fluorescent, but once eluted the organics absorb the UV radiation and leave dark purple spots. That's how most commercial plates work. I guess the
additive mustn't be too hard to prepare and mix in, although you might need something more and in precise concentration.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Baphomet
Thanks for the information guys! Good point about reproducability, Klute. I will mostly be using TLC to compare feedstock spots to product spots, so
if the silica for both plates was produced in the same batch I imagine that would suffice for a simple comparison.
Smuv, thanks for the link. Sounds like a drop or two of PVA is the way to go.. |
Not true, as it variations in thickness are important too. Spot both on the same plate to get around that problem.
As said in the reference, CaSO4 is the common binder. PVA may interact enough to cause problems, or even migrate itself.
|
|
|
jokull
National Hazard
   
Posts: 506
Registered: 22-2-2006
Location: Everywhere
Member Is Offline
Mood: Ice glassed
|
|
All of the above pointed out by Klute should be enough.
Just as a personal comment, the way I used to develop TLC was:
1. To prepare a isopropanol/silica slurry (with a consistency similar to that of Pepto Bismol  ) )
2. To spread such a slurry over a common flat glass
3. To dry the plate in air or into an oven (isopropanol flashes at 15ºC)
If you prepared the slurry in the correct way, your dried plate should not have cracks!
4. To perform the separation with suitable solvents
5. To reveal the plate within a container with some iodine crystals (2 minutes app.)
6. To observe the revealed plate under black light (this way you can see spots that maybe you did not only with iodine).
TLC is quite useful when you know how 
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I must agree, TLC is a very useful tool when experimenting or even just optimizing reactions when you can't acces cheap GC or HPLC use...
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
chemrox > Paper only works for a subset of eluates.. good for pigments but not much else as far as I know
not_important > Thanks for the tip. I will try that..
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
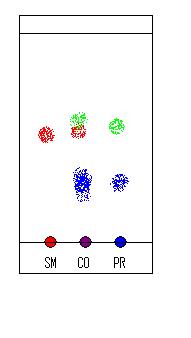
Even when using commercial plates, it's a good idea to not only spot the samples on the same plate, but to run a co-spot as well, because even on a
single TLC plate, the thickness, evenness of packing, etc. can vary enough to give strange results. What this means is, if you want to run say one
starting material vs. the reaction mixture, you need three spots - one spot for the starting material, one for the mixture, and a third spot (usually
placed between the first two on the plate) which has both the starting material AND the reaction mixture (ie. one on top of the other). You can then
be sure of what you're seeing, even if the spots don't line up properly across the plate, because the same two spots are present in the same "lane".
I've attached a diagram to try and make this clearer - "SM" is the pure starting material (which, for convenience, is a red compound), "PR" the
product mixture (blue), and "CO" the co-spot (shown in purple to suggest that the spot is a mixture of the two!). You can also see that the product
mixture contains something green, which has almost the same Rf as the starting material. Without a co-spot, you'd never be able to tell there was a
third compound there, because the Rf's are so close. With one, though, you can see that they don't quite line up!
Developing TLC plates can be done in many ways, and you should choose a developing agent that suits the compounds you expect to see. If you have
fluorescent plates, the first thing to do is view them under short wave UV - because it takes the least time and does not have any chance of
destroying the plate. But, because many compounds do not quench the fluorescence of the plate, it's a good idea to use some sort of stain.
For some good, simple recipes for TLC stains, check out http://holivo.pharmacy.uiowa.edu/separation/stains.html. For a much longer list (with a few alternative concoctions!), try http://www.emdchemicals.com/analytics/literature/TLC_Visuali...
Iodine is nice because it's generally reversible - you just let the I2 evaporate from the plate, and you can then treat it again with another stain.
It's best for olefins, but will also work as a reasonably general stain.
KMnO4 is the stain we use most in our lab. It's very general - it'll stain anything with an oxidisable functional group, and a lot of things without
one! It's also very easy to make.
Anisaldehyde and vanillin are also fairly good general stains, which should be easy enough to get hold of the reagents for.
|
|
|