| Pages:
1
2 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
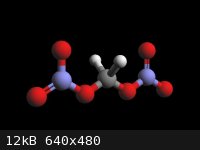
Methylene Dinitrate
Formaldehyde hydrates to Methylene Glycol which nitrated at 5 ºC gives
Methylene Glycol Dinitrate but decomposes violently at 10 ºC , as far as it gets.
http://www3.interscience.wiley.com/journal/114237605/abstrac...
Info on the Dinitrate CAS 38483-28-2
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1236...
http://webbook.nist.gov/cgi/cbook.cgi?ID=38483-28-2&Unit...
http://www.nextbio.com/b/search/ov/Methylene+Dinitrate?id=51...
http://chem.sis.nlm.nih.gov/chemidplus/ProxyServlet?objectHa...
- Nitrooxymethyl nitrate (IUPAC name) what a stupid name
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Regarding this post of this thread _
http://www.sciencemadness.org/talk/viewthread.php?tid=1172#p...
and the cited paper _
Detonation Properties of Explosives containing Nanometric Aluminum Powder
http://www.intdetsymp.org/detsymp2002/PaperSubmit/FinalManus...
and regarding my reply immediately following _
http://www.sciencemadness.org/talk/viewthread.php?tid=1172#p...
citing from the same source _
Nanometric Aluminum Powder Influence on the Detonation Efficiency of Explosives
http://www.intdetsymp.org/detsymp2002/PaperSubmit/FinalManus...
Read page 11 first two paragraphs of - DISCUSSION AND CONCLUSION -
Within reason , for all practical purposes admixed aluminum particle size doesn't matter.
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
http://www.aiexplosives.com/inspections_articles.asp?id=23
Gives an overview of common liquid explosives already discussed in other threads
.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
interesting?
How bout ... Tetra silverdinitridooxosulfate (VI)
"Prepn is by addn of .... under red light."
Stumbled upon in in PATR 2700 [S 111] last night.
djh
----
His talk was like a stream, which runs
With rapid change from rocks to roses;
It slipped from politics to puns,
It passes from Mahomet to Moses;
Beginning with the laws which keep
The Planets in their radian courses;
And ending with some precept deep
For dressing eels, or shoeing horses.
Winthrop Mackworth Praed
The Vicar
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Of interesting commercial explosives nitroguanadine is a good example.
Possibly one of the easiest of many to mfg; has been used by military for many decades (both alone and as TBSP)....and it is truly a "cool explosive".
Otherwise similar to TNT in many ways, it is difficult to work with as the needle characteristic is very tough.
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
I've been making some NQ some time ago. If you can get the educts (most likely as a fertilizer) and use silica gel as catalyst the prep is rather easy
if you take the risk and melt AN and hold it at 160°C for couple of hours....
But I think it's somewhat useless, because you need some real booster to get it detonated and that's what I don't have....but the needles still look
beautiful 
As far as I know NQ isn't used as a standalone for militar purposes, but often in mixture of NCand as it is smokeless and has a lower heat of
explosion than NC and NG, which reduces erosion of the muzzle or however you call it in English ('m german)
But on this board it's not discussed very often (but Engager (or someone else) has a nice prep method.
Haven't seen any video on youtube of NQ detonation either.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Update to the above post - Methylene Dinitrate
Chemistry & Technology of Explosives vol 2 by Urbanski
Synthesis as shown on page 155

www.chemicaldictionary.org/dic/M/Methylene-glycol-dinitrate_...
confirms properties as stated in Urbanski
Boiling point : 75 - 77 °C at 20 mmHg
Density : 1.54 g/cc at 17 ºC
www.chemspider.com/Chemical-Structure.11859.html
additional property data
Boiling Point : 192.8 °C at 760 mmHg
Density : 1.657 g/cc ( this is likely frozen )
http://webbook.nist.gov/cgi/cbook.cgi?ID=C38483282&Mask=...
gives heat of formation as endothermic
* Note
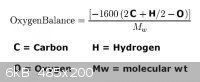
To protect this material from hydrolysis , it can be absorbed into
one quarter it's weight in activated charcoal. The positive oxygen
balance will additionally provide 3 mols of CO ,
CH2(NO3)2 + 3 C => CO2 + 3 CO + H2O + N2
_________________________________________________
Speculation
The prospect of forming Methylene Dinitrite is intriguing.
I find it is an unknown compound. Unlike other alcohols as Methyl ,
Ethyl , Ethylene Glycol and Glycerine that require strong acid or a high
temperature to form an ester, Formaldehyde is a gas in solution that
can esterfy at mild conditions. Sodium Nitrite dissolved in cold 37 %
formaldehyde , later adding cold 30% HCl , obtaining what is surely a
high velocity explosive by the use of over the counter reagents ,
involving almost no chemistry.
2 NaNO2 + CH2(OH)2 aq / + / 2 HCl aq => 2 NaCl + 2 H2O + CH2(ONO)2
Rosco Bodine councils caution with the organic nitrites.
www.sciencemadness.org/talk/viewthread.php?tid=6395#pid72904
.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
How can the BP of MDN be 192°C when it decomposes violently above 10°C? O.o
[Edited on 3-4-2013 by Adas]
Rest In Pieces!
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
36 nitrogen atoms.. drooling?? yes.
we should get this book up on this site tho.. sounds like a interesting book (;
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Adas  | | How can the BP of MDN be 192°C when it decomposes violently above 10°C? O.o[Edited on 3-4-2013 by Adas] |
If the 10 °C is reliable ( I did not retain a reference for this )
it refers to the nitration mixture , which we all know in the
preparation of alkyl nitrates can readily exotherm unless the
nitration is done at cold temperature.
.
As far as the hypothetical dinitrite I propose , similar
structures give a clue to expectations. Methylene diazide
is sensitve but stable. Dinitromethane is not particularly
sensitive but is unstable and readily hydrolyzes unless
it is in the form of a salt. Geminal dihydroperoxides are
very sensitive and unstable , readily hydrolized and
polymerized.
.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Nobody has the balls to try it even on test-tube scale?  Many people have
paraformaldehyde, HNO3 and H2SO4, I guess. Many people have
paraformaldehyde, HNO3 and H2SO4, I guess. 
Rest In Pieces!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
2 NaClO4 + CH2(OH)2 aq/ + / 2 HCl aq => 2 NaCl + 2 H2O + CH2(ClO4)2 / / => CO2 + 2 HCl + 3 O2
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
R E D U X
Reviewing previous postings I've noted that my conjecture on the facile formation of Methylene Dinitrite overlooks important considerations with regards to the expected outcome with the proposed synthesis.
The problem lies with the fixing of Formaldehyde ( CH2O ) in a usable form.
Formaldehyde is supplied in commerce as Formalin in aqueous solutions 37% , 44% , 50% unstabilized or stabilized with 12-15% Methanol. Here's the
problem , the flamable gas Methyl Nitrite is also formed spontaneously from Methanol and 30 % HCl
and NaNO2. This can be avoided using the commercial aqueous only solution. If it cannot be sourced it can be made by generating formaldehyde gas
heating paraformaldehyde to 140 ºC and running that into a receiver with cold water.
( Methyl Nitrite ) with Sulfuric acid instead
www.youtube.com/watch?v=kUOuHpsY8N4
The other big problem is Formaldehyde also reacts with Hydrochloric acid to form highly toxic bis( Chloromethyl ) ether.
https://en.wikipedia.org/wiki/Bis(chloromethyl)_ether
HCl can be avoided by substituting Oxalic acid ( it is used to catalyze polymerization of Formaldehyde with Phenol to form Phenolic resin and itself
remains unchanged.) The resulting Sodium Oxalate has low solubility.
Same thing goes for the proposed Methylene DiPerchlorate ester shown in the preceding post.
The resulting Methylene Dinitrite remains only hypothetical as there exists no citation for this the
simplest of nitrite esters. Just for fun if you Google methylene dinitrite the results will prompt you to " Search instead for methylene dinitrite ". Click on that and the first result is this thread. That alone tells you everything you need to know.
Ethylene Dinitrite however is a known compound
https://pubchem.ncbi.nlm.nih.gov/compound/17952161
Extensibly described here by forum member Rosco Bodine
www.sciencemadness.org/talk/viewthread.php?tid=6395
US2166698 patent , Process of making nitrite esters of polyhydroxy aliphatic compounds
https://patents.google.com/patent/US2166698
https://patentimages.storage.googleapis.com/f3/86/4e/dcf8ec0...
or here _
www.pat2pdf.org/patents/pat2166698.pdf
.
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
Vomaturge
Hazard to Others
  
Posts: 285
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
I've been wondering for a while, is there any report (even theoretical) on propylene glycol di perchlorate (the perchlorate analog to pgdn?)
My googling has only shown ethyl perchlorate and a ton of articles on lithium perchlorate solutions in propylene carbonate (an important aspect of
battery chemistry it seems.)
Incidentally, a back of the envelope calculation shows that a perfect mixture of oxygen and propane should deliver about 8.5 kj/gm, and there is a
small (and very cold) temperature window were both should exist as a liquid. Unfortunately, the two materials are only sparingly soluble. Does anyone
know of a liquid fuel that is miscible (or soluble in stoichiometric quantities) with liquid oxygen?
Edit: reference https://www.researchgate.net/publication/281921173_Solubilit...
"Propane solubilities in oxygen determined were 2.24 % at 110 K and 5.70 % at 120 K"
That's enough to be worrying if it got introduced accidentally, but hardly enough to be useful.
[Edited on 25-1-2020 by Vomaturge]
I now have a YouTube channel. So far just electronics and basic High Voltage experimentation, but I'll hopefully have some chemistry videos soon. |
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Vomaturge  | | I've been wondering for a while, is there any report (even theoretical) on propylene glycol di perchlorate (the perchlorate analog to pgdn?) [Edited
on 25-1-2020 by Vomaturge] |
I recently posted about this here _
www.sciencemadness.org/talk/viewthread.php?tid=1081&page...
For some reason they skipped Propylene , but given that all the related others have been made it shouldn't be a problem.
Ethylene Diperchlorate , Propyl Perchlorate , Isopropyl Perchlorate , Tetramethylene Diperchlorate.
That full paper is here , thanks to Mako.
www.sciencemadness.org/talk/viewthread.php?tid=62326&pag...
Quote: Originally posted by Vomaturge  | Incidentally, a back of the envelope calculation shows that a perfect mixture of oxygen and propane should deliver about 8.5 kj/gm, and there is a
small (and very cold) temperature window were both should exist as a liquid. Unfortunately, the two materials are only sparingly soluble. Does anyone
know of a liquid fuel that is miscible (or soluble in stoichiometric quantities) with liquid oxygen?
[Edited on 25-1-2020 by Vomaturge] |
Explosives of this type are called Sprengel Oxyliquits
https://en.wikipedia.org/wiki/Oxyliquit
.
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
Vomaturge
Hazard to Others
  
Posts: 285
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
I've heard of oxyliquit. I was thinking more about the specific scenario of a stoichiometric solution of a flammable liquid or gas in liquid oxygen.
That would lead to a shorter reaction time, vastly thinner reaction zone, better control of the fuel oxygen ratio, higher density, and much higher
VOD, than simply absorbing liquid oxygen into a solid fuel. I don't know how to calculate it, but I suspect that the greater specific energy released
by such a solution would more than compensate for having a lower density than other explosives. It might have a higher VOD and peak pressure than
PETN.
I just calculated the energy output again, and it looks like 44gm propane to 160 gm of oxygen would be the ideal ratio, and since propane has a lower
heating value of 46.35 kj/gm according to Wikipedia, that gives a total energy release of about 10kj/gm. But liquid propane oxygen mixtures will never
be capable of a high VOD, because most of the required propane will not dissolve and it would be a suspension, rather than a solution. A miscible
cryogenic fuel would likely double or triple the VOD, and give a corresponding increase in brisance.
That paper was very fascinating. I knew of a few alkyl perchlorates, but didn't realize such a wide range of them had been created. If all of those
have been made, I wouldn't be surprised if PGDP were possible after all! Thanks for sharing!
I now have a YouTube channel. So far just electronics and basic High Voltage experimentation, but I'll hopefully have some chemistry videos soon. |
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
The only prospects for your vision are _
https://en.wikipedia.org/wiki/Propene
https://en.wikipedia.org/wiki/1-Butene
Miscibility of these with O2 is not evaluated. Both have a density much less than LOX. Separating into phases is possible.
Methane or Ethane both melt at the boiling point of LOX
https://chemistry.stackexchange.com/questions/111355/can-a-s...
Practical considerations
https://books.google.com/books?id=bsK7vQvNv8wC&lpg=RA5-P...
https://books.google.com/books?id=tDPoBwAAQBAJ&lpg=PA23&...
Same paper you cite from researchgate online here _
www.aiche.org/conferences/aiche-annual-meeting/2007/proceedi...
.
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
Vomaturge
Hazard to Others
  
Posts: 285
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
| Quote: | | [Quote]...it was found that liquid CO and CH4 form clear homogeneous solutions with liquid O2 over the whole composition range from 0 to 100% O2.
|
I just had to bump this old thread to showthis. It looks like the results of a stoichiometric oxygen methane solution were not quite as impressive as I had hoped. The calculated heat of
reaction, VOD, CJ-pressure and peak temperature were 11Mj/kg, 6.01 km/sec, 6.8 GPa, and 5830 K. The actual results were in the 5 km/sec range.
The temperature and energy are pretty high, given that HNB is estimated at around 5500K, HMX, PETN, RDX, and NG are all estimated to be in the 4300-4600 range, and NM and TNT are in the 3400-3800
range.
I think the combination of all triatomic combustion products and low density (880mg/cm^3) are the reason for the weak performance. I wonder what the
far field blast effects are like, since it's 11Mj/kg with all gaseous products. I would expect that the distance for say, 200kPa overpressure is
probably comparable to a TNT charge of over twice the weight...
[Edited on 24-1-2021 by Vomaturge]
I now have a YouTube channel. So far just electronics and basic High Voltage experimentation, but I'll hopefully have some chemistry videos soon. |
|
|
| Pages:
1
2 |