Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
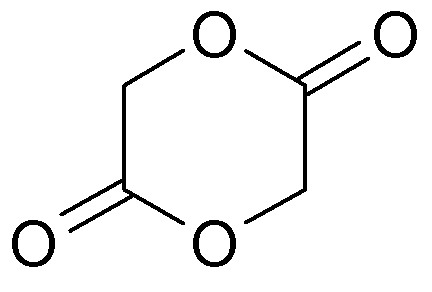
Oxidation of p-dioxan to Glycolide
Glycolide, or diglycolide is the cyclic dimer of glycolic acid. Properly it is 1,4-dioxan-2,5-dione and thus is a double lactone.
Many of you are familiar with the use of TCCA to oxidize THF to GBL for purposes of indoor sports.
Well, glycolide is quite expensive $59 g $230 US Acros) and hard to prepare from glycollic acid which prefers to oligomerize. So I am thinking that a
TCCA oxidation of the preformed cyclic double ether would be much better.
Anyone have any thoughts on tyhis?
[Edited on 31-1-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | Originally posted by Sauron
For purposes of indoor sports.
Anyone have any thoughts on tyhis?
|
Forgive the meanderings Sauron, but looking at your stats and your considerable short-term accumulation of posts, I get this picture of some doddery
old guy reliving "past synthesis glories" from a semi-permanently-occupied rocking-chair, belting out this stuff with arthritic digits on a dusty old
lap-top.
Indoor sports, indeed!
I'm sure the reality is quite different.
You're probably more the "Mr Deeds" of SM.
Oh, and apologies, all, for missing the target topic by the proverbial mile. . .
[Edited on 30-1-2009 by hissingnoise]
|
|
|
jokull
National Hazard
   
Posts: 506
Registered: 22-2-2006
Location: Everywhere
Member Is Offline
Mood: Ice glassed
|
|
What about starting with glyoxal? I'm not familiarized with the compound you're proposing but I have seen partial oxidations of ethylene glycol
towards glycolic acid via photochemstry, perhaps it would be possible to use a related intermediary step.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Glyoxal will polymerize to give brown crap if you look at it wrong. Assuming you are starting with p-dioxane, I don't see why it wouldn't work.
Pushing it to completion might open her up, so separation vs. yield might be iffy.
The other trick is that it might prefer to be the tautomer (1,4-dioxine-2,5-diol). Heat might crack that to give 2-oxoacetic acid?
The similarity to dihydroxyacetone dimer (cracked to give dihydroxyacetone--it loves to make brown crap) got my attention.
Why not make it from polyglycolic acid oligomers as per this patent (because it's complicated and requires a bomb-reactor ): ):
http://www.freepatentsonline.com/5374743.html
I'll look in on it more later.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Hissingnoise:
Totally wrong!
a) It's a very comfy judge's chair, not a rocker, and
b) It's a dusty desktop tower. Laptops are too dinky.
c) No arthritis. Diabetes, hypertension, peripheral vascular disorder, but no arthritis.
So there!
Nolo contendere to the other charges and specifications.
O3:
Vigorous conditions might open up that ring but TCCA does not require such conditions. For that matter NCS ought to do same trick but I have 20 Kg
TCCA and only a couple Kg NCS.
The Japanese claim that glycolide chlorinates to an intermediate that decomposes with a tert amine to oxalyl chloride.
Note that ethylene carbonate on perchlorination decomposes to same plus phosgene. Yje Japanese claim glycolide, no phosgene.
Ethylene glycol if esterified with an acid that has no alpha carbons e.g. trichloroacetyl chloride, if perchlorinated then decomposes to oxalyl
chloride and regenerates the trichloroacetyl chloride. (a few % of the acid is unrecovered.)
All in the patent lit. All have pros and cons. Glycolide may be a pain. No one wants to have a phosgene side product. Trichloroacetyl chloride is
expensive, hard to ship and hard to make (it loves to fall apart to CCl4. See my chloral thread.)
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Here is a US patent teaching the preparation of glycolide of high purity by the following steps
1. React chloroacetyl chloride and glycollic acid in Et2O to obtain O-chloroacetyl glycolic in 30% yield after distillation. Pyridine is acid
scavenger in the reaction I would consider diethylaniline as alternative as it is non hygroscopic and more easily filtered off.
The O-chloroacetyl glycolic acid is converted to its sodium salt using NaOH in THF.
The Cl-CH2-C(O)-O-CH2-C(O)-ONa is pyrolized to crude glycolide which is then purified by fractional sublimation at 25 C/0.03 torr.
NaCl is byproduct,
Critique: Well, chloroacetyl chloride us easy made if nasty, and glycolic acid is cheap. So the lousy yield may be acceptable in the first stage. The
thermolysis and sublimation look like a job for my Kugelrohr if I can get a pump that will get down to 0.03 torr.
[Edited on 31-1-2009 by Sauron]
Attachment: glycolidepatent.pdf (200kB)
This file has been downloaded 530 times
Sic gorgeamus a los subjectatus nunc.
|
|
|