| Pages:
1
2
3
4 |
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@Ozone: please post the whole apparatus (or I can tomorrow)
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
OK, let's see.
I usually plan the fractionation ahead. The usual, 1 forerun, 1 fraction; two are left for either other fractions or to catch the remainder that comes
over. The header joint is well greased which enables it to be turned easily. You can use collection flasks of various size as you see fit. The spiky
bits on the side of each teat are for the connection of springs which should serve to keep the collector from falling off. I just use Keck clamps.
To the best of my knowledge, the rubber tubing on the bottom of the aspirator serves to "muffle" the water jet coming out of the bottom. This prevents
it from spattering all over the place (from the side *and* when it strikes the bottom of the sink) and making a mess. I've not noticed a difference in
vacuum either way.
OK, I was at a conference all day so I'll put together a sample apparatus for photography tomorrow. [Actually, tomorrow I have day-two of conference
and I am judging science fair thursday. this would have to be done on Friday, then.]
I thought the BSA was a nice touch . .
Moooo,
O3
[Edited on 3-2-2009 by Ozone]
[Edited on 3-2-2009 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
You have to grease the shit out of the joint or put a teflon sleave on it. NB there's an inner part not shown that direct condensate to the lowest
"udder." I intended to post the whole apparatus but my camera ran low on batteries. I will post the pic tomorrow to make this very useful piece of
equipment clear to all. BTW this isn't the only kind of glassware that turns on an ST joined under vacuum. McLeod Gauges are often set up that way.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
I have seen these before although it was three pronged. I believe that the "cow" is attached at the end where one would normally put a receiving flask
and at each udder is a small receiving flask. Distill under vacuum and the fraction you distill falls into the receiving flask pointing down. When
another fraction begins to distill over you turn the cow and the fraction is collected in a separate flask.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
the whole cow setup

"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
superman1451
Harmless

Posts: 6
Registered: 7-11-2007
Member Is Offline
Mood: No Mood
|
|
holy cow!

How is that placed on the apparatus?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I'll post a photograph of a whole assembled apparatus tomorrow evening.
That's a big-ass cow, Chemrox! My setup is a little different. Both of my cows, 24/40 125mL and a 14/20 25mL, are male at the header so I have to use
a "gender-bender". I also do not have the drip tube. While nice, and I want one, mine works pretty well just by gravity (it's not as big, though).
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Thats a pretty cool setup.
Iv seen little pig adaptors but Iv never seen a cow.
Do you ever have problems with different fractions ending up mixing trace amounts in each other from the sides of the receiver?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Okay, here's the whole thing. I might put up a picture of a rig with a vertical condenser tomorrow.
You are going to get trace amounts anyway (this technique is not perfect). It is still MUCH easier to purify a fraction than the whole mix (even with
the tube, "heart cutting" is difficult).
Cheers,
O3
[Edited on 5-2-2009 by Ozone]
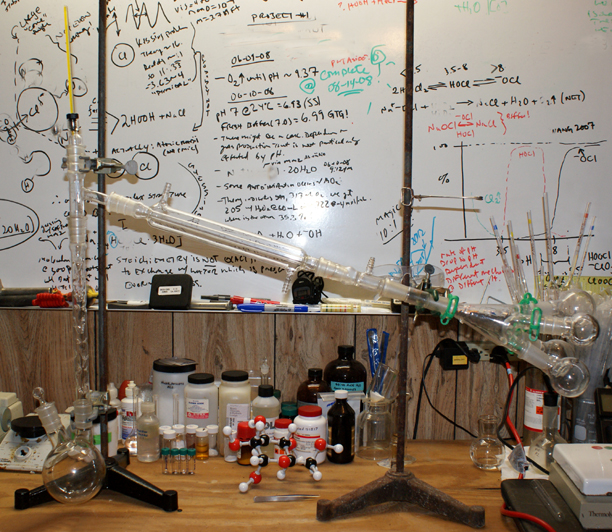
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
A closeup of the vacuum adapter, gender bender and outfitted cow.
If you look closely at the vacuum adapter, you will see where the tube used to be. The 'bender was a tight fit and eventually, the tube broke off . Bummer. . Bummer.
O3

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
taniya.pinkpower1986
Harmless

Posts: 13
Registered: 3-11-2008
Location: pink island
Member Is Offline
Mood: you know
|
|
Practical Vacuum Distillation
Thanks everyone for such nice pics and good infos........................Now it may sound a little stupid.Can I use a hand vacuum pump like this one
in pic

|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Those are for vacuum filtration not distillation. This thread is about practical vacuum distillation. It's simply not practical to stand a set up
pumping away for hours only to pull a slight vacuum, get carpral tunnel and then have the thing break on you.
Get an aspirator or a vacuum pump.
I have an aspirator but no sink in my workspace so I need to totally rearrange my garage before installing a sink and finally vacuum distilling my
nitric acid that has been in my freezer for the past three months.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
get an aspirator or for a little more money an aspirator pump. Both have their uses. An apsirator pump has a water reservoir and a mechanical pump
that pumps water through internal aspirators. No need to run up the water bill but the e bill suffers a bit.
@O3- ours aren't all that different. I went 250 on the flask for the cow and my receivers are 75 ml with 14/20 joints. I forgot to have him put
spring holders on them so I need some 14/20 Klecks. I like having the flow director with the larger volume cow but at 125 you might not need it. I
like having the white board right there where you work!! I have written on the cabinets and windows and parts of the floor...
I noticed you're using a vigeraux. I saw a dramatic improvement when I started using a Snyder column. The distillation I have planned is for
something that boils around 168/0.3 mm so for that I'm using a short path with a 1" built-in vigeraux. I could show the vacuum station but I don't
want her to despair.
[Edited on 10-2-2009 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Yeah, mine's 24/40. I have ~ 1m worth of Snyder ball columns which are very nice to use--but I only really use them for solvent recovery. I find that
it helps to drop some of the solvent into the top of the column (wet the column) prior to use. I suppose I could add a pic of some Snyders tomorrow.
I also have straight wall columns which I pack with steel wool, glass balls, etc. which perform better than the vigreaux. The vigreaux, however...is
easy..and usually works very well. It's a great place to start.
Maybe we should address vacuum manifolds...later.
Nothing like the sound of chattering Snyder columns in the morning!
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
"Nothing like the sound of chattering Snyder columns in the morning!"
hehehe .. yeah that's the best!
I got a three cock manifold made for $45. I can't understand how Ace and Kontes even, charge so bloody much for those things...even with the high vac
cocks. It probably took the glassblower about 10 minutes. I benefit from the guy (my glassblower) being really busy. When it doesn't make it
impossible to get repairs on short notice that is. Like now. I'm working with a new guy I got through the U. We'll see how he does.
[Edited on 11-2-2009 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
I use vacuum setup based on rotary oil vacuum pump, it can provide vacuum up to 0.1 mm. Hg. However setups you shown in this topic look somekind
unusual for me.
1. As far as i can see in provided setups nobody uses manometer or other pressure control. Use of pressure control is absolutely essential because
pressure greatly affects boiling points, if pressure in system will be too low, even high boiling liquid can destill off just about room temperature
and there will be great difficulty to condense it even in long condenser cooled by cold water stream. Before preforming vacuum destilation you must at
first select work pressure range such that lower border pressure will provide high enough boiling point of reaction mixture to condense distillate
efficently and high border pressure that allows to destill your mixture with selected heat source (e.g water or oil bath) at acceptable rate while not
allowing destilled substances to undergo thermal decomposition (for example HNO3 is thermaly unstable). Apropriate pressure range can be selected from
vapor pressure / temperature graph for distilled substance.
2. I don't see boiling capilary in your setups, and this is very strange, because it's absence makes vacuum destillation unstable and even dangerous.
Under reduced pressure gasses that are dissolved in reaction mixture quickly escape from reaction mixture and seed gas bubbles esential for stable
boiling no longer form - liquid can easily become overheated. This is absolutely inacceptable because overheated liquid mixture sooner or later will
boil up with great violence, it can jump over to condenser outlet and in some cases even explosive boil up is possible (heat explosion). To solve this
problem a boiling capilary is used in vacuum setups, this is thin glass tubbing with very small hole in the end that is passed almost to the bottom of
distilation flask, then system is evacuated it passes stream of small air bubbles from atmosphere witch aid phase chance and allow liquid to have
stable boiling. This additional air inlet is also used to fine tune pressure by means of gas valve connected to the top of the inlet capilary.
3. I don't see any trap devices between vacuum outlet and vacuum pump. If you are using water stream vacuum pump if pressure in water system will
suddenly change water from the pump can easily jump into distillate flask and cause some nasty chemical reactions (especialy if destilled substances
react violently with water). If you are using oil vacuum pump some uncondensed fumes can reach pump oil and dissolve in it to lower maximum vacuum, or
if fumes are aggressive they can react with oil or corrode parts of vacuum pump. In usual setups there are trap flasks between vacuum outlet and
vacuum pump. In case of water stream pump trap serve as emergency drain in case of water stream pressure fluctuations, and in case of oil pumps this
trap flask is usualy strongly cooled (for example with solid CO2) to freeze any vapours not condensed by condenser and not allow them to enter pump
oil or leave evacuated system.

[Edited on 22-3-2009 by Engager]
|
|
|
Gruson
Harmless

Posts: 30
Registered: 22-10-2005
Location: Southern Netherlands
Member Is Offline
Mood: Creating!
|
|
Since I intend vacuum distilling and use of a rotary evaporator with an oil pump as well (<1mbar), I was wondering if I could replace the dry
ice/acetone cold trap with an 60 cm graham condensor. (water on the outside) This would be cooled with an refrigerated circulator bath, this one http://www.capovani.com/dp/cat/107/63160/iinfo.cfm?LCl=986&a... to be more precise. It would be running with water/EG at -5 till -8 degrees
celcius. Is this sufficient to prevent solvent from reaching the pump oil? The rotavap/liebig cooler would also be cooled with this temperature.
It could be that the purpose of your life is only to serve as a warning to others.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
That all depends on what you intend to distill. Look up a graph on the BP of the solvent with pressure plotted in, and cross-check that with the
temperature of the condensing water and the efficiency of your condenser.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by Gruson  | | Since I intend vacuum distilling and use of a rotary evaporator with an oil pump as well (<1mbar), I was wondering if I could replace the dry
ice/acetone cold trap with an 60 cm graham condensor. (water on the outside) This would be cooled with an refrigerated circulator bath, this one http://www.capovani.com/dp/cat/107/63160/iinfo.cfm?LCl=986&a... to be more precise. It would be running with water/EG at -5 till -8 degrees
celcius. Is this sufficient to prevent solvent from reaching the pump oil? The rotavap/liebig cooler would also be cooled with this temperature.
|
Remember that although something may be well below its boiling point for a given pressure it still may have some, even significant, vapour pressure
and as such will eventually end up in your pump.
For most common solvents this is not really problematic as your can bleed them from your oil easily enough (assuming a vane pump here). Especially if
using a fully synthetic vac pump oil. However for distillations of more reactive substances you really want to avoid any contamination into your pump
and -8 ain't gonna cut it even for moderate vacuums. That is why the dry ice cold finger is standard good practise, however its annoying for the home
experimenter because you must have the dry ice around and it has this silly habit of subliming away and warming up the earth.
I posted recently in relation to small domestic benchtop ice cream makers being an excellent alternative to the dry ice cold finger. They run
continuously (i leave mine on all week), sit at around -38 and negate the need for the consumable dry ice. I just fill mine with ethanol and immerse
the trap into it.
|
|
|
Gruson
Harmless

Posts: 30
Registered: 22-10-2005
Location: Southern Netherlands
Member Is Offline
Mood: Creating!
|
|
That is an excellent idea. I presume you have the icemaker with built-in compressor? Which type exactly? (I believe you have a "Nemox"?) I can get my
hands on dry ice, but that would be a 70 km drive. Okay for one particular synthesis, but I was looking for a solution on the long term. Does ethanol
has the heat capacity needed for efficiently cooling the cold trap?
It could be that the purpose of your life is only to serve as a warning to others.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by Gruson  | | That is an excellent idea. I presume you have the icemaker with built-in compressor? Which type exactly? (I believe you have a "Nemox"?) I can get my
hands on dry ice, but that would be a 70 km drive. Okay for one particular synthesis, but I was looking for a solution on the long term. Does ethanol
has the heat capacity needed for efficiently cooling the cold trap? |
The reason i leave it on all week is it has about a 20min warm-up time (ha funny to describe it as this) before it is at it lowest temperature. This
solution is not as good as dry ice obviously, but many more times convenient and permanent. I use ethanol because i have it and didn't want to use any
solvents potentially aggressive to the polymers of the unit, whatever they may be. If your unit is fully cooled it works fine.
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
A great OTC method I've adapted uses a Reynolds Handi-Vac, hand held vacuum pump, and have had decent results. Not the same as my carbon vane, but it
seems to be able to deal with the organic vapors so far, and is handy and easy to grab real quick. Maybe not for critical sensitive work, but surely
to speed things along which otherwise are slow, but not necessarily all that heat sensitive without it.
|
|
|
marksev1
Harmless

Posts: 26
Registered: 16-3-2009
Member Is Offline
Mood: No Mood
|
|
What about the crushed ice with calcium chloride hexahydrate in ratio 2:3, i read that it achieves temp. about -40 degrees celsius...would that be
sufficient (for example for DCM)? And also calcium chloride is quite cheap.
[Edited on 7-4-2009 by marksev1]
|
|
|
Shingoshi
Harmless

Posts: 49
Registered: 20-4-2008
Location: Salem, Oregon
Member Is Offline
Mood: No Mood
|
|
Would this be the right thread to ask questions about boiling butane (or ammonia) from water using Mazzei Injectors? I can start a new thread if I
need to. I want to know if such aspirators are capable of the task?
Here's the information on these injectors:
http://www.mazzei.net/products/injector_info.htm
Shingoshi
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Is your goal to purify the water, or to recover the butane/ammonia?
For simply removing the gas, bubbling air through the water might do the job, use a packed column with counterflow of air and water.
|
|
|
| Pages:
1
2
3
4 |