| Pages:
1
2 |
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Detectable radioactivity from rubidium?
Naturally occuring rubidium exists as about 72 % of Rb-85 which is stable, and 28 % of Rb-87 which has a half-life of about 50 billion years.
I was wondering if rubidium salts are radioactive enough to detect. So I did some research:
According to "Half-Life and Beta Spectrum of Rb-87", K. F. Flynn; L. E. Glendenin, Phys. Rev. Vol 116(3) 1959 pp. 744-748, the
maximum beta decay energy is about 272 keV.
Half life is 50 billion years. That's about 5x1019 seconds.
1 mole of rubidium chloride (121 g) contains about 5x1023 atoms of rubidium.
So there should be on the order of 103 beta decay events per second in a mole of RbCl.
Now, I expect these beta particles to generate X-rays by Bremsstrahlung given that rubidium is a somewhat heavy atom. Copper (molar mass 63.5 Da),
molybdenum (96 Da), rhodium (103 Da) are used as targets in X-ray tubes, so I think Rubidium would behave in the same way.
Does anyone have a geiger counter and rubidium-containing substances on hand to test whether the radioactivity can be detected? I have a Geiger
counter (SBM-20 tube, sensitive to beta and gamma radiation) but no rubidium, as its quite expensive.
As a side-note I have successfully detected radiation from 500 g of potassium chloride (beta decay at around 1.3 MeV and gamma at 1.5 MeV) using the
SBM-20 tube.
Feel free to move the thread to radiochemistry if appropriate, although there is no real chemistry here.
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
I've tried with an ampoule of rubidium metal and rubidium iodide, but did not observe a clear increase in counts.
In the case of the ampoule of metal, the glass was not very thick, but it would probably still block most of the beta.
The rubidium iodide was in air with the GM tube almost touching it. The iodide ions probably block some of the beta and x-rays.
I have not tried counting for extended periods of time (my counter is analog). I don't know the model of my GM tube, but it is easily able to pick up
the activity of potassium salts. When I hold the tube above any potassium salt, the increase in count rate is immediately obvious.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
mhhh i have a digital geiger counter with an SBM-20 tube, and i couldn't detect any counts above background near my 5kg of potassium nitrate, anyway
i'll try again tomorrow as i come back home from the holidays
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Spock
Harmless

Posts: 22
Registered: 27-3-2014
Member Is Offline
Mood: Slightly Radioactive
|
|
It looks like it's a fairly weak beta particle with an average energy of 81.67keV. I'd recommend an end window tube or organic scintillator probe.
I'll have access to quite a few decent detectors including a liquid scintillation counter in a week and a half, all I need to do is find some
rubidium.
|
|
|
Texium
|
Thread Moved
4-1-2019 at 07:57 |
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Interesting, so the beta is too easily stopped. If I get around to buying a rubidium salt I'll try to pick something light, not as likely to shield
the Bremsstrahlung (nitrate or chloride).
Ubya I should state that in my case the potassium salt was in a thin plastic bag. The probe was placed in a fold of the bag to surround it with
potassium. The max rate I got that way was about 300 CPM (about 10 times background). The dose rate drops to normal at a few tens of centimeters
Spock be sure to post your results if you can detect anything!
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Heptylene  |
Ubya I should state that in my case the potassium salt was in a thin plastic bag. The probe was placed in a fold of the bag to surround it with
potassium. The max rate I got that way was about 300 CPM (about 10 times background). The dose rate drops to normal at a few tens of centimeters
|
i'm home now, and i tested again, maybe when i checked a few years ago i used a smaller quantity of potassium nitrate or maybe i just put the probe
too distant. i tried again putting the GM tube between 2 plastic bags each full with 1kg of KNO3, and i got 240CPM when my background is around 60CPM
so yea potassium 40 hello
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Livebyagreenhouse
Harmless

Posts: 1
Registered: 19-1-2019
Member Is Offline
|
|
(I lost access to the spock account)
I received 3.5g of RbCl in the mail today and I was able to get a detectable increase in counts over background. I was using a Ludlum 44-7 end window
probe attached to a scaler.
The probe was less than 1cm above approximately 1g of RbCl spread out in a circle. The average of three one minute background counts was 28.6666cpm.
The average increase over background of three counts with the RbCl under the detector was 44cpm.
Simply putting the detector at the mouth of the open bottle caused no detectable increase in count rate.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Wow great news, thanks for the update!
|
|
|
Blarglesworth
Harmless

Posts: 7
Registered: 1-2-2019
Member Is Offline
|
|
In order to detect Rb-87, you pretty much have to use something that can pick up alphas - its weak beta won't penetrate all that much more material
than an alpha would. I've gotten a very good response - greater than the response to KCl, gram for gram - using an Inspector EXP with a pancake probe
sensitive to alphas.
Now for a real challenge: indium. Most of it is In-115, a beta emitter with a half-life of about 440 trillion years! Crunching the numbers, there
should be about 15 decays a minute in each gram of it. Using a foil covering the whole probe surface and 12-hour count times I was able to attain
statistical significance three times in a row compared to identical 12-hour counts with the foil removed. I was quite amazed that I managed to get
anything. Of course I can't prove that there wasn't some sort of trace Th/U/K contaminant or something, but the extra counts I got were right in line
with what I was expecting.
I also managed to get lanthanum-138 with a gamma scintillator, surrounding it with lots of La2O3 and running for several hours. There was a very
slight but still significant bump right at its energy. Its half-life is 105 billion years, a good >4000x shorter than In-115, but its abundance is
only 0.09%.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
So the way I remember it is, a year is pi times 10^7 seconds. This is a surprisingly good approximation. 50 billion is 5 * 10^10, so that gives me 5
pi * 10^17 = 1.6 * 10^18 seconds.
You seem to have taken 1 year = 10^9 seconds, which is off by a factor of 30 or so.
| Quote: | Half life is 50 billion years. That's about 5x10^19 seconds.
1 mole of rubidium chloride (121 g) contains about 5x10^23 atoms of rubidium.
So there should be on the order of 10^3 beta decay events per second in a mole of RbCl. |
Off-by-one error, it should be 10^4, although in this case it's actually going to be about 3.6 * 10^5 because of the factor of 30 from earlier.
Curiously, this gives you almost exactly 10^3 events per gram-second.
[Edited on 2-2-2019 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
There aren't any.
|
|
|
Spock
Harmless

Posts: 22
Registered: 27-3-2014
Member Is Offline
Mood: Slightly Radioactive
|
|
You're right, there aren't any alpha particles. However your detector needs to be sensitive to particles with a penetrating capability not too unlike
that of an alpha particle. In practice that means a mica window gm tube.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Or, you can pick up the accompanying gammas.
As you say, the betas never get very far so the beta radiation measured at the surface is pretty much the same from a gram or a ton.
But the gammas are much more penetrating and so using a larger sample is more effective.
I have measured the radiation from a 1 Kg bag of potassium chloride with a geiger counter.
Even allowing for the fact that geiger's don't react efficiently to gammas, I still think most of the counts were gammas, rather than betas.
If you really want to do it, try a suitable scintillation detector.
Very roughly 40K is about 10 times more active than 87Rb on a weigh for weight basis and it's about 0.012% of the natural material, rather than about
28% for rubidium
Natural rubidium should be markedly more active than natural potassium on a gram for gram basis
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Gram for gram is not too relevant when measuring decay. As Geigers don't measure gamma radiation too well and beta doesn't travel too well volume is
more relevant than weight.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | | Gram for gram is not too relevant when measuring decay. As Geigers don't measure gamma radiation too well and beta doesn't travel too well volume is
more relevant than weight. |
RbCl is about 140% of the density of KCl.
Given the other approximations, that difference can probably be ignored.
The higher Z means it's got higher stopping power too, but that's also not a big effect.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Oops you're right! My bad. I actually remember that 30 years is about 1 billion seconds, I don't know how I mixed that up with 1 year...
[Edited on 3-2-2019 by Heptylene]
|
|
|
Blarglesworth
Harmless

Posts: 7
Registered: 1-2-2019
Member Is Offline
|
|
I put together a spreadsheet with all the radionuclides with half-lives between 700 million and 10^18 years. Using its abundance and half-life, I calculated the
activity due to that nuclide for a sample of the element. Note that this doesn't include decay chains.
Rb is far more active than K: 863 Bq/g compared to 31. The difficulty is in picking up the fairly weak betas with a maximum energy of 283 keV. It
doesn't emit gammas, unlike K, and I've had no luck at picking up brehmsstrahlung radiation from them hitting thicker-walled GM tubes. So it pretty
much requires a mica window detector sensitive to alphas.
The only elements that have a nuclide with more activity than 1 Bq/g of the element are U, Th, Re, Rb, Sm, Lu, and K. Rb-87 has the fourth-most
activity weighted by its abundance, following U-238, Th-232, and Re-187.
The decay of Re-187 is pretty much undetectable by the amateur, because it has one of the lowest beta energies (if not the lowest) of all known
nuclides - only 2.7 keV max, far less even than tritium. Sm-147 decays by an alpha with low energy (2.2 MeV); I'm not sure if this even gets through
mica windows. So basically only Rb, Lu, and K are easily detectable - and again Rb still pretty much requires a mica window or similar.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I wonder if scintillation cocktails might pick up something.
2.7KeV isn't a lot by nuclear standards, but in principle, it's a lot of visible photons.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | I wonder if scintillation cocktails might pick up something.
2.7KeV isn't a lot by nuclear standards, but in principle, it's a lot of visible photons. |
2.7 keV is probably enough to activate an e-beam phosphor used in CRTs. Maybe placing the sample against a CRT TV phosphor screen would work.
|
|
|
Spock
Harmless

Posts: 22
Registered: 27-3-2014
Member Is Offline
Mood: Slightly Radioactive
|
|
I doubt you'd make it through the glass of the screen. Of course you could break open the TV tube.
I'd certainly expect a liquid scintillation cocktail to work though, does anyone know of a home built LSC? It probably wouldn't be unreasonably costly
or difficult to do, especially if the builder already had a detector that could drive a PMT.
|
|
|
Ormarion
Hazard to Self
 
Posts: 54
Registered: 19-12-2017
Location: France
Member Is Offline
Mood: Alkylating her DNA
|
|
If i can give my experience, i "think" i was able to detect it. I tried to measure the activity of 100g of RbCl from an old lab using my gamma scout
(that have 3 digits) and over a period of 30min i got an approximative différence of 0.015 µSv/h, so yea, really not that much.
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Only 27% of natural Rubidium is Rb87 which decays to Sr87 with a half life of 50 billion years and a beta 282Kev, much like C14 it`s the perfect
candidate for liquid scintillation .The Sr/Rb ratio is used to date certain rocks, I can try but I cannot make any promises.
|
|
|
Cezium
Harmless

Posts: 39
Registered: 5-1-2015
Member Is Offline
Mood: No Mood
|
|
Indium
Quote: Originally posted by Blarglesworth  | In order to detect Rb-87, you pretty much have to use something that can pick up alphas - its weak beta won't penetrate all that much more material
than an alpha would. I've gotten a very good response - greater than the response to KCl, gram for gram - using an Inspector EXP with a pancake probe
sensitive to alphas.
Now for a real challenge: indium. Most of it is In-115, a beta emitter with a half-life of about 440 trillion years! Crunching the numbers, there
should be about 15 decays a minute in each gram of it. Using a foil covering the whole probe surface and 12-hour count times I was able to attain
statistical significance three times in a row compared to identical 12-hour counts with the foil removed. I was quite amazed that I managed to get
anything. Of course I can't prove that there wasn't some sort of trace Th/U/K contaminant or something, but the extra counts I got were right in line
with what I was expecting.
I also managed to get lanthanum-138 with a gamma scintillator, surrounding it with lots of La2O3 and running for several hours. There was a very
slight but still significant bump right at its energy. Its half-life is 105 billion years, a good >4000x shorter than In-115, but its abundance is
only 0.09%. |
I got tested 200g of my Indium metal at our Faculty of Nuclear Science and Physical Engineering and they couldn't pick up any detectable radiation.
Got around 100g or so of RbCl, 1g of Rb metal and access to various scintillation probes and GMs so maybe will try to measure it some day...
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Quote: Originally posted by Blarglesworth  |
I also managed to get lanthanum-138 with a gamma scintillator, surrounding it with lots of La2O3 and running for several hours. There was a very
slight but still significant bump right at its energy. Its half-life is 105 billion years, a good >4000x shorter than In-115, but its abundance is
only 0.09%. |
How small is your detector? La138 is usually pretty easy to pick up.
this is 1 hour of acquisition with a 3"x3" NaI detector
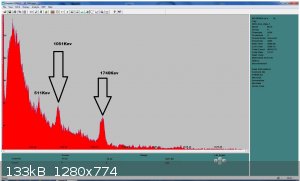
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Using the liquid scintillation counter, I was able to detect about 300 counts per minutes (~5Bq) of Rb87.
Starting with 1g of RbCl diluted to 100 mL of DI water to get a 10,000ppm solution which is mixed with the scintillation cocktail (ecolume) at a ratio
of 3 to 1.
The count yielded about 80 for the background and just over 300 for the Rubidium.
here is the Beta spectrum.
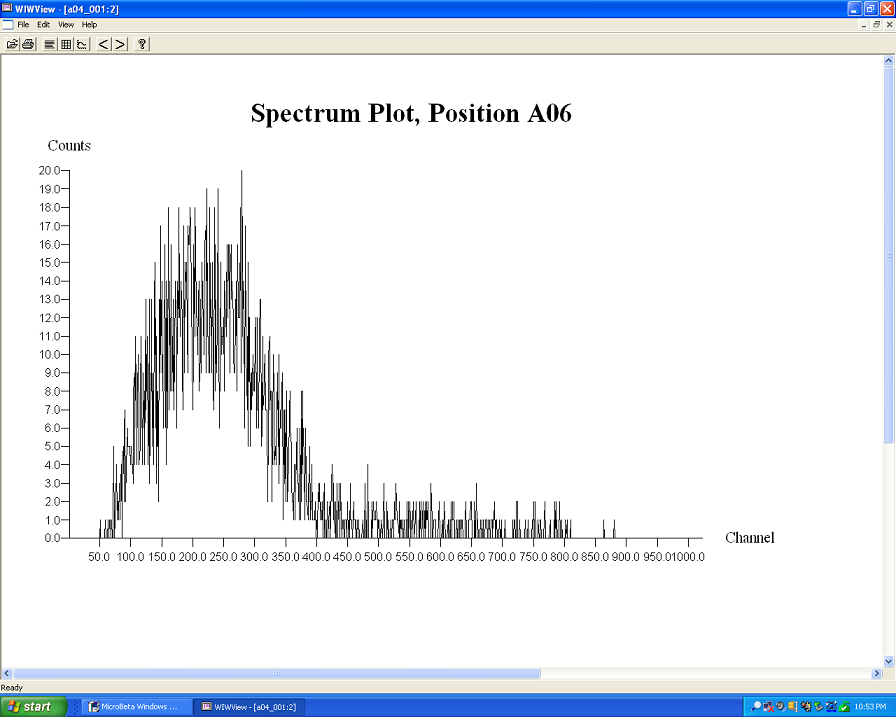
Calculating the activity for Rb87 in about 0.01 g at the time of "creation" gives 276Bq
Now just for shits and giggles and since we have a pretty good natural clock I thought about estimating the age of the Rubidium atoms by comparing the
activity i calculated and the one i measured.
And I got 13.68 billion years ... Now that is some spooky shit right there...
I know Rubidium is a primordial nucleus and it had to be formed during a supernovae before the solar system existed . But this level of precision is
beyond remarkable and suspiciously accurate.
In the end, the only think to note here is the order of magnitude, the exact number does not really matter. If I had gotten 100 trillion years or 53
milliseconds then i know something would have been very wrong. ..
I will probably put a video together with all my math so it can be checked out . I am finishing up with other details I`d like to include .
Still pretty cool right ?
|
|
|
| Pages:
1
2 |