| Pages:
1
2 |
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I have a question: How did you determine the refractive index (and with such accuracy??!). Also, what is your use for the benzyl chloride? You know it
may be easier to have chlorinated toluene using NCS? The reagent itself isnt difficult to prepare either.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I use my refractometer (bought of ebay for £30  ) similar to this one: ) similar to this one:
http://cgi.ebay.co.uk/Abbe-Refractometer-0-95-Brix-Refractiv...
Edit- i might be off by 0.001 on the las digit but hey, its still pure enough for anything i am gonna use it for!
I am attempting the synthesis of cyclomethycaine. I will use the benzyl chloride to add the cyclohexoxyl group via hydrogenation, which will in turn
hydrogenate 2-methylpyridine.
[Edited on 26-12-2009 by Picric-A]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
DJF90 there is nothing easier than pouring benzyl alcohol, conc HCl into a separating funnel and shaking! Easy preparation of benzyl chloride. Note
well that benzyl chloride is known not to be stable and tends to be stablized by addition of a small % trimethylamine!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I know this panziandi, but it you have to go through the hassle of making the benzyl chloride first then why not start with something as abundant as
toluene; NCS is a little harder to come by and so will have to be made, or you can us an alternative reagent also under free radical conditions. I've
made t-butyl chloride like that also, very simple and gives the product in good yield. Also worth noting is that benzyl alcohol can react with HCl via
two pathways, Sn1 and Sn2, but both give the same product!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
DJF90 - As mentioned earlier in the post, i have just bought ~2 litres of benzyl acetate, BDH A.R. and thought i would use it for something.
Of course chlorinating toluene is simple to go form A-->B however you do have to seperate the isomers...
As Panziandi says, making Benzyl chlroide from the alcohol is simple! so instead of a chlorination apparatus i just mixed them in a rbf. shook for
around 5 mins then stirred on a mag. stirrer for 10 followed by seperation of the benzyl chloride.
Thanks for the trimethylamine info panziandi!
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
For reaction of benzyl alcohol with HCl(aq) you can read here:
http://www.sciencemadness.org/talk/viewthread.php?tid=5839&a...
Even at 1:3 (alcohol:acid, m/m) ratio, conversion to chloride is about 75%.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Thanks for the link Kmno4, indeed i will never get 100% yield however i will recover the Benzyl alcohol and re-react that, so no alcohol is wasted.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
How do you want to recover benzyl alcohol ? Distillation ?
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Yes distillation, a few HCl fumes may come over and dissolve in the BzOH but that doesnt matter as the ultimate product is BzCl.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
This methode of separation can give very poor results, because of sensitivity of benzyl alcohol to acids. Anyway, I do not want to be a bad prophet,
hah.
BTW. Mixing benzyl chloride with trimethylamine easily gives benzyltrimethylammonium chloride :
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv4p0098
Using any amine as stabilizer is rather bad idea.
If benzyl chloride is free from traces of acids, it is completely stable at room temperature.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
So vigerous washing with dilute Na2CO3 (aq) should make it stable?
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
The commercial product from aldrich is stabilized with a very small amount of propylene oxide.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
hmm, hydrolysis of propylene chlorohydrin... that seems doable!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Hydrolysis? What? Its an intramolcular nucleophilic attack of oxygen on the beta carbon to displace chlorine, forming the oxide (epoxide). You'll need
Ca(OH)2 as a base to form the alkoxide of the chlorohyrin to act as the nucleophile.
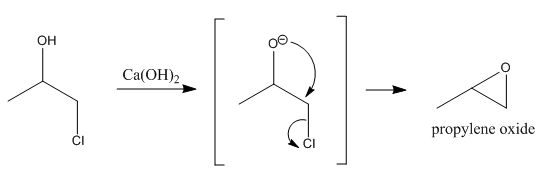
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Yea sorry, i forgot how to describe it so i just said hydroylysis, knowing a base is needed, sorry
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I can think of many reactions where a base is used but is not a hydrolysis. Failing to use the correct terminology causes confusion. I'm sure you can
find a procedure in Vogel or another practical text. Note how Ca(OH)2 is used as the base; this is not just a random selection, although the reason
escapes me at this present moment in time.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I was not proposing that you should inhibit it with propylene oxide, I was showing that even very pure BnCl must be inhibited for long term storage.
Epoxides are very carcinogenic and very toxic, I would think twice before making any volatile epoxide.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Of course smuv, one should not prepare anything of such nature without appropriate equipment/apparatus, and a knowledge of what they are doing.
Perhaps styrene oxide could be used as a safer alternative. However, Panziandi has already stated that his bottle of benzyl chloride is stabilised
with a small % of trimethylamine, so I'd also advise that!
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by smuv  | | Epoxides are very carcinogenic and very toxic, I would think twice before making any volatile epoxide. |
The
carcinogenicity of of epoxides is due to their undergoing epoxide ring-opening reactions with the amine groups on proteins which comprise DNA, to
which they become bonded via N atoms, thereby preventing the DNA from functioning properly. Epoxides can get into the body via the polynuclear
aromatic hydrocarbons, e.g. benzpyrene, in soot and especially tobacco smoke; these undergo enzymatic oxidation in the liver of an end benzo ring to
form a cyclic diol epoxide, which then reacts with -NH2 groups. McMurry's Organic Chemistry (in References) has more details of this.
[Edited on 29-12-09 by JohnWW]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yes John, thanks for that; if I'm interested in the carcinogenicity of epoxides I'll go read a book...
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
ok, ok chill... i think we all know the basics on epoxides and as such we all know not to mess with em - a remider on that once in a while is good
for us though
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You shouldnt really mess with anything. Synthesis under controlled conditions and with suitable apparatus is *slightly* different...
|
|
|
| Pages:
1
2 |