| Pages:
1
2
3 |
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Ethylene diamine perchlorate
I made a funny compound, which burns with a beautiful pink flame.

I made this by taking 5 ml of 10% HClO4 and adding drops of an approximately 10% solution of ethylene diamine in water. I added around 1 ml of this
10% solution and then I added single drops. After each two or three drops I tested a tiny sample of the liquid with some NaHCO3 and if it still
fizzled I added another drop of 10% ethylene diamine solution. I stopped adding ethylene diamine until the solution does not cause fizzling of NaHCO3
solution. In this situation there probably is a slight excess of ethylene diamine. In the process of sampling I have taken good care not to
contaminate the liquid with NaHCO3.
Next, I poured the liquid in a hour glass and put it in a warm and dry place for a few days. This results in formation of a pale yellow solid, which
still seemed slightly wet (no nice crystals but some sticky semicrystalline mass was produced).
When a small quantity of this sticky mass is put on a small spatula and kept in a flame then it first does some sputtering and bubbling and then at
once it gives an intense flame of fire with a pink color.
The picture shows the end of the burst of fire (at the most intense point the camera was strongly overexposed).
I'll make a webpage with more pictures and more details but I already wanted to share this nice result with you.
[Edited on 26-12-09 by woelen]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Betaine would probably also be an interesting titrant 
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This is the webpage in which the experiment is described in more detail. You can also see a video demonstrating the reaction.
http://woelen.homescience.net/science/chem/exps/NH2CH2CH2NH2...
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
- very good woelen
It has a lot of merit for a " funny compound ", it is a serious explosive
( lead block performance 144 % to that of TNT ) as are all organic base
prechlorate adducts
See Federov , Page E 237 - Ethylenediamine Diperchlorate
The chelating property of Ethylenediamine also lets it make coordination
complexes with oxidizer salts.
See Federov , Page E 235 - Ethylenediamine Complexes
PHILOU Zrealone commented here _
http://www.sciencemadness.org/talk/viewthread.php?tid=10058#...
" Stangely hydrazine perchlorate is less powerfull than the nitrate, in the other cases
perchlorates are always higher performers on all aspects; ( maybe due to excess oxygen
content.) "
Rosco Bodine commented here _
http://www.sciencemadness.org/talk/viewthread.php?tid=4076#p...
" Mixtures of urea perchlorate , hexamine diperchlorate , and ammonium perchlorate
in some combination of binary or perhaps tertiary mixtures could also result in some
interesting compositions."
- on Methylamine Perchlorate , which is very similar
http://www.sciencemadness.org/talk/viewthread.php?tid=4076#p...
http://www.sciencemadness.org/talk/viewthread.php?tid=6791#p...
Related threads & post
Hexamine Diperchlorate
http://www.sciencemadness.org/talk/viewthread.php?tid=364
Urea Perchlorate
http://www.sciencemadness.org/talk/viewthread.php?tid=3286
TriperchloroTriaminobenzene
http://www.sciencemadness.org/talk/viewthread.php?tid=1081&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=1081&a...
.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I made another somewhat related compound, N(CH3CH2)4ClO4. This can be made by dissolving tetraethylammonium bromide, N(CH3CH2)4Br, in water and adding
a concentrated solution of a soluble perchlorate to this. I used 20% HClO4, but a concentrated solution of Mg(ClO4)2 or NaClO4 also will work. When
the solutions are added to each other, a dense white somewhat flocculent precipitate is formed.
Next, I boiled the solution for a while and all of the precipitate dissolves when this is done. I continued boiling for a while so that appr. 50% of
all water evaporated. Next, I allowed the liquid to cool down for two hours or so. After this, the liquid is filled with many needle-shaped crystals.
The wet mass of crystals and liquid were put on a sintered glass filter and the mass is rinsed three times on this filter with ice cold distilled
water. Next, I put some paper tissue on the bottom side of the sintered glass filter, such that most of the liquid is absorbed. Then I scraped off the
solid material and put it in a petri dish in a warm dry place for a few hours. The result is a purely dry feather-like solid which is not hygroscopic.
The latter surprises me, because tetraethylammonium bromide is very hygroscopic.
When the material is kept in a flame, then it does not ignite at once. You have to keep it in the flame for a while just like the ethylene diamine
perchlorate. But after a while it sets off white a deep orange flame and a strong whoosh sound, and a cloud of black soot is ejected from the
explosion. The soot can be explained, because there is insufficient oxygen in the oxidizer. This material is less powerful than the ethylene diamine
perchlorate, but the visual effect of setting off this compound is nice. I'll try to make a video of this tomorrow.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It has been some time since I made this compound, but the material is not really energetic. I made a video, but it is not really worth posting here.
Just some crackling and sputtering, that's all. I can understand this, because the compound has four ethyl groups for roughly 4 O-atoms and there is a
very strong oxygen deficiency in this compound.
I now also have tertramethyl chloride and with this compound I made [N(CH3)4]ClO4. This compound is much more energetic and much easier to ignite than
[N(CH3CH2)4]ClO4. I also made the compound [N(CH3)4]IO4 and the latter is even easier to ignite, but the reactions seems to be somewhat less powerful.
The smoke effect of [N(CH3)4]IO4 is nice though. It gives a mix of purple and yellow smoke. I already made a video of this and that video will follow
soon.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Here is the promised video. It took some time, but I still think it is nice to share this with others. It is the burning of [N(CH3)4]IO4. This
compound is ignited very easily and gives a lot of yellow smoke and purple iodine vapor on burning.
http://www.homescience.net/chem/exps/NH2CH2CH2NH2_HClO4/N(CH...
Here follows a single frame of the yellow smoke reaction:

This amazing amount of smoke is from just a few cubic mm of solid material!
___________________________________________________________________________
In the meantime I also made hydrazine perchlorate, N2H5ClO4. This compound also is quite interesting. You can read about that on my website as well.
http://woelen.homescience.net/science/chem/exps/hydrazine_pe...
Most special are the fairy-powder effects when boiling N2H5ClO4 sprays little droplets into the air, which explode with tiny specks of light and a
crackling/hissing noise.

[Edited on 17-6-10 by woelen]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Woelen: you might try the BH3NH2NH4ClO4 that is described in "stronger than Astrolite" post. The BH3 is much less reactive after it has become an
adduct. There is also exists the di-nitrate of hydrazine, and therefore the di-perchlorate could probably be made.
Anyone wanting to make HClO4 might try first crystallizing out NH4ClO4, which has a low solubility in water (less than NaClO4),
and then reacting NH4ClO4 with chlorine gas, being careful to not use too much Cl2, otherwise NCl3 will form and cause an explosion. 2NH4ClO4 + 3Cl2
--> N2 + 6HCl gas + HClO4
If water is not used, one might get pure HClO4
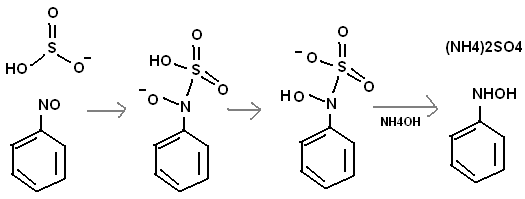
I do not know why there is so much interest in N2H4 and not NH2OH. If there is some organic group attached to it, NH2OH can be more poweful than
hydrazine, with much less toxicity. I believe that ONCH2CH2NO will react with bisulfite to form HONHCH2CH2NHOH, the sulfate of that compound anyway.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
BH3NH2NH3ClO4 sounds like an interesting compound to play with, but making this is beyond my capabilities. I have no borane and cannot make it from
chemicals available to me (remember, I am not a professional chemist, but am doing this as a hobby, as most of us do here).
Making the diperchlorate of hydrazine is not easy at all. I tried this, but the result is a wet and sticky mess and no crystals are obtained. This is
what I also wrote in my webpage. In air, even at a warm place, the material simply does not want to dry.
I have the impression that N2H6(ClO4)2 and N2H5ClO4+HClO4 are in equilibrium with each other and in air, this mix is EXTREMELY hygroscopic. Maybe it
can be prepared when it is dried in vacuum at somewhat elevated temperatures over P4O10 and/or H2SO4, but under such extreme conditions, the anhydrous
HClO4 also is volatile and simply leaves the compound, or it ignites the whole mess. On the other hand, the mono-perchlorate is perfectly stable and
non-hygroscopic.
The idea of making HClO4 from NH4ClO4 unfortunately does not work in practice. In the past I have done similar experiments with this. I attempted to
make H2SO4 from (NH4)2SO4 (purified fertilizer, so-called "zwavelzure ammoniak" as we can buy it in the Netherlands). This reaction is not smooth at
all. Cl2 and NH4(+) ion can coexist in aqueous solution fairly well and in no way is there a smooth reaction in which N2 and HCl are formed. Just try
it yourself. At high concentrations of ammonium ion, indeed NCl3 can be formed. So, this method is not practical and gives a shitload of all kinds of
side reactions, which are slow.
I do have some NH2OH.HCl and I could try mixing a highly concentrated solution of this with 30% HClO4. If hydroxylammonium perchlorate also has
relatively low solubility like ammonium perchlorate, I might be capable of isolating this from the liquid. I'll try that next weekend. If I obtain
some dry powder from this, I could see how energetic this is. I do not expect too much of this, because of the really large excess oxygen balance in
this compound. On the other hand, the endothermic character of NH2OH may add to the energetic properties of NH2OH.HClO4. To be continued....
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Hydroxylamine perchlorate would be LESS powerful than the hydrazine salt, however it is a better oxidizer, and can be more powerful if than the
hydrazine salt if there are other groups to oxidize, or even if mixed with CH3NO2 to make a "cheddite".
Buy boric acid (available in many pharm. stores). Heat it to make B2O3. Burn with magnesium in the absence of air. You will get some magnesium boride.
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I tried making the hydroxylammonium perchlorate. It is not that difficult to make, but it is not as easy as making N(CH3)4ClO4. The salt is soluble
quite well in water and is not easily crystallized. I did the following:
Dissolve some NH2OH.HCl in a little amount of water. Some heating was required to get all of it dissolved.
To this, I added some 50% HClO4. When this is done, no crystals appear. Apparently NH2OH.HClO4 is soluble very well.
Next, I boiled down the solution, until the liquid became somewhat syruppy. I didn't want to go any further, because of fear of sudden violent
decomposition. While boiling down, a lot of HCl-gas escapes!
On cooling down, crystals of NH2OH.HClO4 separated. I put the crystalline mass on a sintered glass fritte and sucked away liquid with a piece of
tissue paper on the other side of the glass fritte. Next I rinsed the piece of paper with a lot of water (just to be sure no dangerous paper/HClO4
mixes are formed) and threw away the wet piece of paper. On the glass fritte a fairly dry white crystalline mass remains.
The crystals do not become really dry, they remain somewhat sticky, but they are useful for experimenting.
I put some of the crystals on a spatula and kept this above a flame. When this is done, the solid melts and starts sputtering and then suddenly it
burns with a blue flame. The reaction is only marginally less energetic than the reaction of hydrazinium perchlorate.
I indeed can imagine that if a methyl group could be attached to the hydroxyl amine, then a more energetic compound can be prepared.
[Edited on 19-6-10 by woelen]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
And how to methylate NH2OH? Maybe start with the methyl group to begin with. I think HSO3- might reduce a nitroso group.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
One might be able to do a "nitrosoation" on toluene with conc. H2SO4 and NaNO2. This would make CH3C6H2(NO)3.
Use the reaction in the above post with HSO3- and one might get
CH3C6H2(NHOH)3. This could be used to make an energetic salt.
[Edited on 24-6-2010 by Anders Hoveland]
[Edited on 24-6-2010 by Anders Hoveland]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
I dont think so. Nitrous acid on aniline would give you the diazonium salt which would decompose into phenol and other things very easily. Also conc
H2SO4 and sodium nitrite would give very conc HNO2 which would rapidly decompose into nitrogen oxides. The way I would do it is mononitrate benzene,
then reduce with Zn and NH4Cl to phenylhydroxylamine. That could be oxidized using sodium or potassium dichromate to the nitrosobenzene. Alternatively
you could oxidize aniline to nitrosobenzene.
But I dont even know why we are talking about this, the thread is on ethylenediamine perchlorate and related compounds...
woelen, interesting expiriments! Have you tried methylamine perchlorate? I was going to attempt this once I get some more conc HCl to make perchloric
acid, but money is kinda tight right now. I have a feeling methylamine perchlorate would be pretty soluable, but also could be a good energetic
oxidizer when mixed with other fuels.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Referring to the previous posting above in this thread of this topic here _
http://www.sciencemadness.org/talk/viewthread.php?tid=13174#...
Amine base + acid explosophore adduct compounds applying this theme
are quite varied and a prolific area for investigation , as has been observed
before by me here _
http://www.sciencemadness.org/talk/viewthread.php?tid=10058#...
and here _
http://www.sciencemadness.org/talk/viewthread.php?tid=14033#...
NOTE * N,N-Dimethyl-2,4,6-Trinitroaniline is not basic enough to protonate
as Nicodem pointed out.
1,3,5-triaminobenzene
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6695...
http://cdb.ics.uci.edu/cgibin/ChemicalDetailWeb.py?chemical_...
Can readily be combined as a perchlorate salt and also with Trinitromethane
http://www.sciencemadness.org/talk/viewthread.php?tid=1081&a...
2 C6H3(NH2)3 • 3HClO4 => 3 CO2 + 9 CO + 9 H2O + 6 HCl + 3 N2
C6H3(NH2)3 • 3HC(NO2)3 => 3 CO2 + 6 CO + 6 H2O + 6 N2
Protonation of 1,3,5-triaminobenzenes in aqueous solutions
http://pubs.acs.org/doi/abs/10.1021/ja00230a034
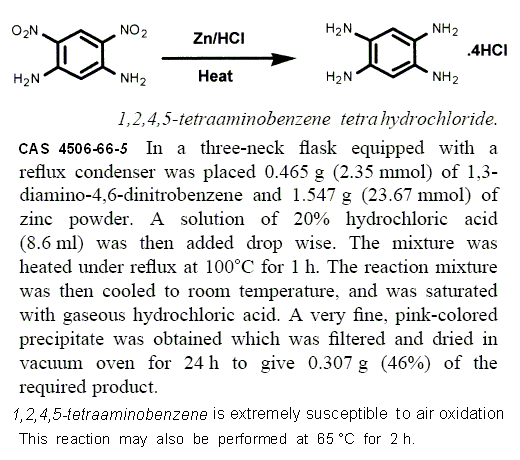
1,2,4,5-Tetraaminobenzene
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=7826...
http://cdb.ics.uci.edu/cgibin/ChemicalDetailWeb.py?chemical_...
The same as the triamino shown above but affords better oxygen balance.
It is readily oxidized in air as described in the attached excerpts , but
stable as the hydrochloride salt in which it is commercially available.
C6H2(NH2)4 • 4HClO4 => 5 CO2 + CO + 5 H2O + 4 HCl + 2 N2
C6H2(NH2)4 • 4HC(NO2)3 => 7 CO2 + 3 CO + 7 H2O + 8 N2
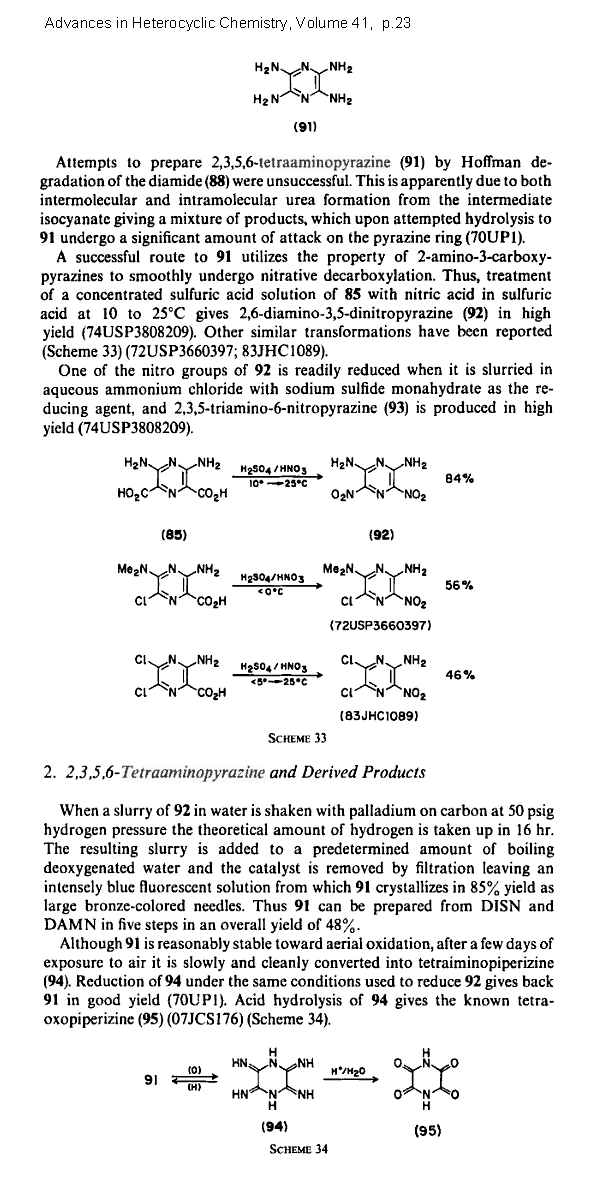
2,3,5,6-Tetraaminopyrazine
This is a recently isolated heterocyclic investigated mostly for polymer applications.
Due to a higher nitrogen content replacing carbon , a nearly oxygen balanced nitrate salt
makes sense. The rest have a positive oxygen balance.
C4N2(NH2)4 • 4HNO3 => 2 CO2 + 2 CO + 6 H2O + 5 N2
C4N2(NH2)4 • 4HClO4 => 4 CO2 + 4 H2O + 4 HCl + 3 N2 + 2 O2
C4N2(NH2)4 • 4HC(NO2)3 => 8 CO2 + 6 H2O + 9 N2 + O2
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
2,3,5,6-Tetraaminopyrazine
This patent dates to 1974 so this material has been around for a while after all.
http://www.google.com/patents/download/3808209_DI__NITROPYRA...
2,3,5,6-Tetraaminopyridine Hydrochloride Preparation
http://www.mdpi.com/1420-3049/14/5/1652/pdf
2 C5HN(NH2)4 • 4HNO3 => CO2 + 9 CO + 13 H2O + 9 N2
4 C5HN(NH2)4 • 4HClO4 => 20 CO2 + 18 H2O + 16 HCl + 10 N2 + 3 O2
2 C5HN(NH2)4 • 4HC(NO2)3 => 17 CO2 + CO + 13 H2O + 17 N2
The WiZard is In
made this related post on previous work on this theme here _
http://www.sciencemadness.org/talk/viewthread.php?tid=4697#p...
______________________
Alternatively the amines of the precusors can be replaced directly with nitro groups
2,3,5,6-Tetranitropyrazine
Theoretical Study on Polynitropyrazines and Their N-oxides
Available in the references section
http://www.sciencemadness.org/talk/files.php?pid=184713&...
2,4,6-Trinitropyridine & Related Compounds
Available in the references section
http://www.sciencemadness.org/talk/files.php?pid=112877&...
High Nitrogen Explosives Part 1 - 2,6-Dinitropyridines & Dibenzo-1,3a,4,6a-Tetraazapentalenes
http://handle.dtic.mil/100.2/ADA285388
re-directs to :
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA285388&Locati...
.
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
If I am not mistaken, the compound in figure (92) can be oxidized with concentrated trifluoracetic acid and H2O2 to add an oxide to the heterocyclic
nitrogen on the top, forming (after neutralization) a very insensitive explosive with ideal properties.
This was described on a site called "Energetic..." something, but now I cannot find it.
"Advances in Energetic Materials"?
http://www.scribd.com/doc/17087769/A-Review-of-Energetic-Mat...
http://pubs.acs.org/doi/abs/10.1021/ol049076g
"DNTZ" from Organic chemistry of explosives By Jai Prakash Agrawal, R. D. Hodgson
looks interesting, while the book is 150USD, the contents are irresistable and fascinating for anyone with a passion for advanced energetic materials.
DNTZ could be thermally stable, despite the two nitro groups, because the amine would be expected to be electron donating, through the aromatic
resonance
On p15 of "Oxidation and Nitration of C-N bonds", for instance, it discusses gem-nitro groups, saying that oximes can be turned to dinitro with nitric
acid and ammonium nitrate, but that then the psuedonitrole product needs treatment with H2O2 (or NO2) to yield the gem-dinitro
Bis-(triaminoguanidinium)-5,5′-azotetrazolate
[Edited on 12-9-2010 by Anders2]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Heterocyclics available as base adducts with oxy acid groups is extensive.
Examples of basic media include :
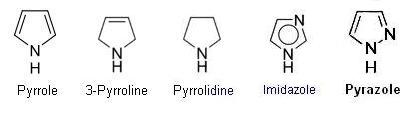
- 5 member rings with one nitrogen, ranging from aromatic to saturated,
. such as pyrrole, 3-pyrroline, pyrrolidine.
- 5 member rings with two nitrogens, ranging from aromatic to saturated,
. such as Imidazole, pyrazole, 2-pyrazoline, pyrazolidine.
- 5 member rings with three nitrogens, such as 1,2,3-triazole, 1,2,4-triazole.
- 5 member rings with four nitrogens, tetrazole.
- 6 member rings with one nitrogen, ranging from aromatic to saturated,
. such as pyridine, piperidine.
- 6 member rings with two nitrogens, ranging from aromatic to saturated,
. such as pyridazine, pyrimidine, pyrazine, piperazine
- 6 member rings with three nitrogens, ranging from aromatic
. 1,2,3-triazine, 1,2,4-triazine, 1,3,5-triazine, to saturated, Triazacyclohexane
- 6 member rings with four nitrogens, 1,2,3,4-tetrazine, 1,2,4,5-tetrazine
Additionally any of the rings containing an attached amine group
can also serve as base adducts with oxy acid groups.
Examples of these basic cyclic compounds include
- aminopyridine, diaminopyridine, triaminopyridine,
- aminopyridazine, diaminopyridazine, triaminopyridazine,
- aminopyrimidine, diaminopyrimidine, triaminopyrimidine,
- aminopyrazine, diaminopyrazine, triaminopyrazine,
- aminotriazine, diaminotriazine & triaminotriazine ( melamine )
Many of these have already been subject of investigation as propellants.
Substituted nitro derivatives of the rings is an ongoing effort for the
development of new explosives compounds.
Studies of Aminonitropyridines, Aminonitropyrimidines, and Their N-Oxides
http://www.mdpi.com/1422-0067/3/8/858/pdf
Synthesis, Characterization and Explosive Properties of
3,5-Dinitro-2,4,6-Triaminopyridine and Its 1-Oxide
http://handle.dtic.mil/100.2/ADA299641
Re-directs to:
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA299641&Locati...
[Edited on 15-9-2010 by franklyn]
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
I found the link. page 2, compound "LLM-105"
http://docs.google.com/viewer?a=v&q=cache:YUiQqbN8BYsJ:p...
Note that LLM-116 is stated to be calculated as having 90% the power of HMX. Now compare the with the molecular structure of DNTZ. This indicates that
DNTZ is likely more powerful than HMX, and possibly more stable, since the nitrogens will serve as an electron donor through the aromatic ring.
"Vogl has patented an explosive prepared from ethylenediamine perchlorate, by forming an addition compound with picric acid."
(Perchlorates: Their Properties, Manufacture And Uses) p139
Message to the Moderators: Perhaps move some of these posts to the topic "New Energetic Materials - Current Research", starting with franklyn's post
(which started with "2,3,5,6-Tetraaminopyrazine...This patent..."). Things started slowly drifting a little off topic before that, but the posts
before franklyn's related to ethyldiamine perchlorate at least to some extent.
[Edited on 14-9-2010 by Anders2]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
nitrocholine perchlorate
It isn't clear if this material has any value or not and there is very little in the literature about it. The synthesis would seem simple enough but
the hazards of synthesis are also unknown.
Choline forms a perchlorate salt , mp 273C
C5H14NO-ClO4
The perchlorate salt on being heated in water bath with 65% HNO3 does form a nitratoperchlorate mp 186C ,
sl sol water , explodes strongly when heated above mp .
C3H9N(ClO4)CH2CH2-ONO2
This compound is also called nitrocholine perchlorate .
Hofmann , K.A. and Hobold , K. , Ber. chem. Ges. 44 , 1766 (1911)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
possible Mannich reaction ?
This " hypothetical " ( exist ? ) compound has been puzzling me a bit lately so I am going to just throw it out there for consideration and comment.
No search of the literature has been done to learn more about this possible reaction and no experiments either .....so this is purely a "shot in the
dark" kind of an idea for a compound and a reaction route that may or may not work for the compound that also may or may not exist. Please humor me
. Does this reaction work ?
It involves a Mannich reaction for nitromethane and formaldehyde and ammonium perchlorate :
CH3NO2 + HCHO + NH4ClO4 ----> CH2NO2CH2NH2 - HClO4 + H2O
As for nomenclature, perhaps the desired theoretical compound CH2NO2CH2NH2 - HClO4 would be called nitroethanolamine perchlorate
I can't find any reference to such a compound.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | It involves a Mannich reaction for nitromethane and formaldehyde and ammonium perchlorate :
CH3NO2 + HCHO + NH4ClO4 ----> CH2NO2CH2NH2 - HClO4 + H2O
As for nomenclature, perhaps the desired theoretical compound CH2NO2CH2NH2 - HClO4 would be called nitroethanolamine perchlorate
I can't find any reference to such a compound.
|
You can not get a nitronate ester like that as the addition of the MeNO2 derived nitronate to the carbonyl is reversible. You can utmost get the
normal Manich reaction product which would be O2N-CH2CH2-NH2*HClO4. However, I found no examples of Mannich reactions between nitromethane and
ammonia. I would expect polysubstitution could and might occur at both sides, at the alpha-nitroalkane position and the nitrogen of ammonia. Already
with normal Mannich reaction, when employing ammonia, one gets substitution products up to the tertiary amine. Otherwise the Mannich reaction with
nitroalkanes is known and has been relatively well studied (for one example, see DOI: 10.1021/jo01019a069).
O2N-CH2CH2-NH2*HClO4 is only described in a patent (abstract bellow). O2N-CH2CH2-NH2 can be prepared via the Michael reaction (see second reference).
| Quote: | Process of preparing 2-nitroalkylamines, preferably in the form of salts. Soroka, Miroslaw; Siwek, Andrzej. (Politechnika
Wroclawska, Pol.). Pol. (2005), 6 pp. CODEN: POXXA7 PL 188456 B1 20050228 Patent written in Polish. Application: PL 98-324914
19980217. Priority: . CAN 144:128653 AN 2006:110575 CAPLUS
Patent Family Information
Patent No. Kind Date Application No. Date
PL 188456 B1 20050228 PL 1998-324914 19980217
Priority Application
PL 1998-324914 19980217
Abstract
The title compds. H2NCH2C(R1)(R2)NO2 [R1, R2 = H, alkyl] or preferably its salts H3N+CH2C(R1)(R2)NO2X- [I; R1, R2 = H, alkyl; X = chloride, sulfate,
perchlorate, etc.] were prepd. by reacting N-(triarylmethyl)-1-amino-2-nitroalkane A3CNHCH2C(R1)(R2)NO2 [II; R1, R2 as above; A = aryl, preferably Ph]
with hydrochloride acid or other inorg. acid in preferably org. solvent at 250-400K. Aq. solns. of I were also prepd. by acidolysis of II with
hydrochloride acid or other inorg. acid in H2O at 250-400K. Thus, treating N-(triphenylmethyl)-1-amino-2-nitroethane with dry hydrogen chloride in
Et2O at 273-310K afforded 100% 2-nitroethylamine hydrochloride.
Aliphatic nitro compounds. VII. Preparation of 2-nitroalkylamines. Heath, Royden L.; Rose, J. D. Imperial Chem. Industries
Ltd., Manchester, UK. Journal of the Chemical Society (1947), 1486-9. CODEN: JCSOA9 ISSN: 0368-1769. Journal language unavailable.
CAN 42:23078 AN 1948:23078 CAPLUS
Abstract
NH3 and primary or secondary amines interact with a-nitro olefins to give 2-nitroalkylamines. Those formed from NH3 or aliph. amines are in general
extremely unstable but can be reduced over Raney Ni to derivs. of (CH2NH2)2. N-(2-Nitroalkyl)arylamines from arom. amines are somewhat more stable
but are best isolated and characterized as their salts. In many cases O2NCH2CH2NO3 (I) can be used as a convenient lab. substitute for O2NCH:CH2 and
Me(O2NCH2)CHOAc (II) or Me(O2NCH2)CHNO3 (III) can replace MeCH:CHNO2 (IV). I (13.6 g.), added dropwise to 20 cc. MeOH satd. with dry NH3 at -5 and
the mixt. stirred 4 h. at -5 to 0, gives 9 g. crude O2NCH2CH2NH2, which forms a black tar in 1-2 h. and does not yield cryst. salts but on redn. in
MeOH gives (CH2NH2)2. IV and MeOH-NH3, stirred 3 h. at 0, give 55% 2-nitroisopropylamine, b10 50-5, decomp. within 24 h. (HCl salt, m. 114); redn.
yields 52% MeCH(NH2)CH2NH2. III (30 g.) in 200 cc. dry ether, treated 1 h. with NH3, gives 12.7 g. bis(2-nitroisopropyl)amine, b0.5 60-2.
MeCH:C(NO2)Me and MeOH-NH3, stirred 2 h. at 0, give 60% 2-nitro-3-aminobutane, b20 75-8, n18D 1.4720, decomp. in 2 days (HCl salt, m. 115 (decompn.),
also unstable); redn. gives 40% [MeCH(NH2)]2. Me2C:CHNO2 in C6H6 gives 40% 1-nitro-2-amino-2-methylpropane, b11 62.5, decomp. in 3-4 days (HCl salt,
m. 182 (decompn.)); redn. gives 75% Me2C(NH2)CH2NH2. I (6.8 g.), added to 7.3 g. Et2NH in 100 cc. ether at 0, gives diethyl(2-nitroethyl)amine, which
could not be distd. at 15 mm. (violent decompn.); HCl salt, m. 72-5; picrate, m. 88. MeCH:C(NO2)Me gives 65% 2-nitro-3-diethylaminobutane, b11, 90-5,
begins to decomp. in 12 h. (picrolonate, m. 267 (decompn.)). I (5.9 g.), added dropwise to 11.8 g. tetrahydroquinoline at a temp. below 30, the mixt.
stirred 1 h., dissolved in 50 cc. ether, and treated with ether-HCl, gives 75% N-(2-nitroethyl)-1,2,3,4-tetrahydroquinoline-HCl, m. 132; CH2:CHNO2
gives 12.5%. I (27.2 g.), added dropwise to 37.2 g. PhNH2 in 250 cc. ether at room temp.
and stirred 2 h., gives 65% PhNHCH2CH2NO2, m. 37; HCl salt, m. 109; Ac deriv., m. 99; with CH2:CHNO2 this yields 50% %Ngr;,N-bis(2-nitroethyl)aniline,
m. 64 (HCl salt, m. 128). PhNHMe and CH2:CHNO2 give 50% N-methyl-N-(2-nitroethyl)aniline, b0.2 110 (HCl salt, m. 82).
N-Ethyl-N-(2-nitroethyl)aniline, pale green oil, b0.1 108, n19D 1.5597; HCl salt, m. 114, 70%; picrate, m. 106; CH2:CHNO2 gives 75% of the HCl salt.
N-(2-Nitroisopropyl)aniline, yellow, m. 33. MeCH:CHNO2 (43 g.), treated dropwise with 53.5 g. PhNHMe at a temp. below 30 and the crude product in 500
cc. ether added to 500 cc. ether contg. 20 g. HCl, gives 92% of the HCl salt, m. 126, of N-methyl-N-(2-nitroisopropyl)aniline, pale green oil, b0.5
112-15; picrate, m. 116; perchlorate, m. 116; HCl salt of p-NO deriv., dark green, decomp. 160; II gives 50% of the HCl salt.
N-Ethyl-N-(2-nitroisopropyl)aniline-HCl, m. 123, 90%. N-Ethyl-N-(2-nitropropyl)aniline-HCl, m. 126, 50%. N-(2-Nitro-1-methylpropyl)aniline, b0.5 86,
n17D 1.5570, 72%; HCl salt, m. 122; perchlorate, explodes on warming. |
Edit: A quote from the above mentioned DOI: 10.1021/jo01019a069 says: "Mannich bases, I (R = H), of nitromethane had not been reported, however, since
all attempts to prepare them had led exclusively to the disubstitution products, II (R = H).[ref. 3-5]" Reference 5, which is DOI:
10.1021/ja01205a004, describes some Mannich reactions on nitromethane giving disubstituted products of the O2N-CH(CH2NR2)2 type. This explains why I
could not find any example of the Mannich reaction between ammonia, formaldehyde and nitromethane. Most likely the condensation would give either a
polycyclic compound, like 3,7,10-trinitro-1,5-diazabicyclo[3.3.3]undecane for example, or some cross-linked polymer or oligomer.
[Edited on 18/10/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Nicodem, Thank you for your insight. I am still thinking about this possible reaction, even though it may be another method for "red goo".
My idea was that the proposed compound is possibly a variant "derivative" of methylamine perchlorate via an
intermediate methyleneimine derived from the initial reaction of the formaldehyde with the ammonium ...
NH4ClO4 + HCHO ---> CH2-NH(HClO4) + H2O
CH3NO2 + CH2-NH(HClO4) ----> CH2NO2CH2NH2(HClO4)
Your rewriting the formula as O2N-CH2CH2-NH2*HClO4
is probably better.
[Edited on 18-10-2010 by Rosco Bodine]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | NH4ClO4 + HCHO ---> CH2-NH(HClO4) + H2O
CH3NO2 + CH2-NH(HClO4) ----> CH2NO2CH2NH2(HClO4)
Your rewriting the formula as O2N-CH2CH2-NH2*HClO4
is probably better. |
You should also rewrite the second product above as it should be O2N-CH2CH2-NH-CH3*HClO4. Also, imines have a double bond, e.g. CH2=NH.
Anyway, that is indeed a Mannich reaction, but like the above mentioned references explain, the Mannich reaction on nitromethane does not stop on the
monosubstitution stage, it instead gives disubstitution (the monosubstituted products can be obtained via the Michael addition instead). Furthermore,
since Mannich reactions with ammonia usually give the tertiary amines I would expect the product of the Mannich condensation of nitromethane,
formaldehyde and ammonia would be the already mentioned 3,7,10-trinitro-1,5-diazabicyclo[3.3.3]undecane (though more likely further condensation with
HCHO is possible and this is probably why there is nothing in the literature). Unfortunately, SciFinder gives no hits on this compound, but it does
give several references for its 3,7,10-triethyl substituted analogue which is obtained by the condensation of 1-nitropropane, formaldehyde and ammonia
(for the synthesis, see DOI: 10.1016/S0040-4039(01)87863-1 and DOI: 10.1039/JR9580002319). 1-Nitrobutane gives the corresponding tripropyl analogue
(with 1-nitropropane or -butane no further condensation with HCHO is possible).
Using primary amines instead of ammonia gives similar tricyclic structures. For example, ethylenediamine can be used to give an interesting compound
(see abstract bellow).
The Mannich reaction between nitromethane, formaldehyde and methylamine is also possible and gives
3,7-dimethyl-1,5-dinitro-3,7-diazabicyclo[3.3.1]nonane (the Mannich base gets cross-linked with an additional HCHO via Henry condensation, though this
might be only due to the basic conditions used). Similar products are obtained by using ethanolamine, benzylamine, t-butylamine and other primary
amines (Russian Chemical Bulletin, 54 (2005) 414-420).
Interesting coordination compounds are obtained by the Mannich reaction of metal glycinates, nitromethane and formaldehyde
(DOI:10.1016/j.poly.2007.03.018).
However, if the purpose is to get something energetic then the Mannich reaction of dinitromethane, formaldehyde and ammonia to potentially give
3,3,7,7,10,10-hexanitro-1,5-diazabicyclo[3.3.3]undecane or its salts would be more interesting. Or the reaction of dinitromethane, formaldehyde and
ethylenediamine to potentially give 3,3,7,7-tetranitro-1,5-diazabicyclo[3.3.2]decane. Or their perchlorate salts as in the spirit of this thread.
Unfortunately, there is nothing in the literature on these.
| Quote: | Reactions of aliphatic nitro compounds. XXI. Products of reaction of 1-nitropropane with formaldehyde and ethylenediamine.
Urbanski, T.; Kolinski, R. Inst. Technol., Warsaw, Roczniki Chemii (1956), 30 201-13. CODEN: ROCHAC ISSN: 0035-7677. Journal written in
English. CAN 51:5540 AN 1957:5540 CAPLUS
Abstract
cf. C.A. 51, 421a. 1-Nitropropane (I) reacts with CH2O (II) and NH2CH2CH2NH2 (III) to give 3,7-dinitro-3,7-diethyl-1,5-diazabicyclo[3.3.2]decane
(IV). The reactions of IV and of its degradation products are described. To 9.8 g. 77% aq. III 50 ml. 30% II was added with cooling and then 22.3 g.
I, and the mixt. kept 24 hrs. The product was a yellow gum and an aq. layer. The gum was dissolved in 75 ml. EtOH and slowly crystd. to give 11.0 g.
crude IV and 23.5 g. brown gum. Pure IV m. 104-6; hydrochloride, m. 140-1. From the aq. layer there was obtained 0.5 g. tertiary amine, m. 137-9
[hydrochloride, m. 212-14 (decompn.)], and 2 g. 6-nitro-6-ethyl-1,4-diazacycloheptane (V) (isolated as the hydrochloride), an oil (hydrochloride,
charring at about 300; picrate, m. 149-51; nitroso deriv., m. 122-3 ). Refluxing IV hydrochloride (3.2 g.) in 96% EtOH gives besides 2 g. unchanged
IV, II, and 0.5 g. 1-(2-nitrobutyl)-6-nitro-6-ethyl-1,4-diazacycloheptane hydrochloride (VI), m. 160-2 (decompn.) (free base and nitroso deriv. are
oils). Heating 2.9 g. IV in 10 ml. concd. HCl gives 1.3 g. V hydrochloride, while ethanolic HCl gives VI in addn. to V hydrochloride. Refluxing 3.1
g. VI in 10 ml. concd. HCl gives a primary nitroparaffin, II, and V hydrochloride. The structure of IV is confirmed by its synthesis from V
hydrochloride and 2-nitro-2-ethyl-1,3-propanediol (VII) in which V and VII react at room temp. in aq. soln. at pH 8. The synthesis of the
1,4-di-p-toluenesulfonyl deriv. of V from ethylene-N,N-di-p-toluenesulfonamide and 1,3-dichloro-2-nitro-2-ethylpropane (VIII) failed. VIII (9.5 g.),
m. 48-50, was obtained by reaction of 15 g. VII in 20 ml. pyridine with 15 ml. SOCl2; 1 g. of cyclic sulfite of 2-nitro-2-ethyl-1,3-propanediol, m.
68-70, was obtained as a by-product.
Conformational behavior of 3,7-dimethyl-1,5-dinitro-3,7-diazabicyclo[3.3.1]nonane. Zefirov, N. S.; Palyulin, V. A.; Efimov, G. A.;
Subbotin, O. A.; Levina, O. I.; Potekhin, K. A.; Struchkov, Yu. T. Mosk. Gos. Univ., Moscow, USSR. Doklady Akademii Nauk SSSR (1991),
320(6), 1392-5 [Chem.]. CODEN: DANKAS ISSN: 0002-3264. Journal written in Russian. CAN 116:127882 AN 1992:127882 CAPLUS
Abstract
The title compd. I was prepd. by the cyclocondensation reaction of MeNH2.HCl, H2CO, and MeNO2 in the presence of NaOH, and its conformation
investigated in the solid state by x-ray crystallog. and in the liq. state by low-temp. 13C NMR. In the solid state the conformation of I was
couch-tub; couch-tub .dblarw. tub-couch conformational equil. was obsd. in soln., with an activation barrier of 6.5 kcal/mol. A barrier of 8.7
kcal/mol was detd. for diazabicyclononanone deriv. II, indicating that the couch-tub .dblarw. tub-couch process is synchronous with N inversion.
|
[Edited on 18/10/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Nicodem  | Quote: Originally posted by Rosco Bodine  | NH4ClO4 + HCHO ---> CH2-NH(HClO4) + H2O
CH3NO2 + CH2-NH(HClO4) ----> CH2NO2CH2NH2(HClO4)
Your rewriting the formula as O2N-CH2CH2-NH2*HClO4
is probably better. |
You should also rewrite the second product above as it should be O2N-CH2CH2-NH-CH3*HClO4.
[Edited on 18/10/2010 by Nicodem] |
You just lost me there ...where is the final CH3 coming from ?
As for the other .... ( amending the bond symbol for methyleneimime )
NH4ClO4 + HCHO ---> CH2=NH(HClO4) + H2O
CH3NO2 + CH2=NH(HClO4) ----> CH2NO2CH2NH2(HClO4)
Possibly the shorter chain would not cyclize and the reaction would proceed if the formaldehyde was added gradually or there was simultaneous addition
so formaldehyde was not present in excess ?
The energetic salt if it exists would be isomeric with ethylnitramine or ethylnitramide (plus perchloric acid)
[Edited on 18-10-2010 by Rosco Bodine]
|
|
|
| Pages:
1
2
3 |
|