| Pages:
1
2
3 |
kafka
Harmless

Posts: 34
Registered: 26-7-2006
Member Is Offline
Mood: No Mood
|
|
lefetamine
it should be clear that no discussion of lefetamines uses shall be discussed as well as any similar compounds.
now, what im looking to discusus is a theoretical synthesis to this compound from easily available chemicals. not for illicit purposes, simply because
of the chalange and enjoyment of reaching the theoroetical goal.
the sought after compound is one of several isomers. a reaction that resulted in a specific isomer would be great but for now anything producing a
reasonable amount will be adiquete.
Lefetamine aka: SPA, santenol as well as (R)-N,N-dimethyl-1,2-diphenyl-ethanamine
a paper discussing the original synthesis has not been found by me. however later papers discussing the production of similar chemicals have given a
few clues to this rather simple chemical.
desoxybenzoin (also seen it spelled deoxybenzoin, mistake or?) goes through a reductive amination with dimethylamine inorder to produce a mix of
isomers. seperation goes on from there.
can Al/Hg along with dimethylamine HCl be used for this step? that would make things much more "OTC" that Na borohydride or whatever.
would that reaction give the desired product? there is also friedel crafts with phenylacetyl chloride and benzene (?). could FeCl3 be used instead of
AlCl3 here? benzene qualififies as OTC or close to it (toluene rxns etc) the phenylacetyl chloride can be made obviously but this route uses some
chems that are popular with another group of products not so popular with the gov...
sticking with the deoxybenzoin what wold be the easiest and most availabe route to it?
Im not familar with most of the chems used to make it and have yet to find common sources for them (not to say there are not any). the reduction of
benzoin (hg electrodes..?), water + bromosibene at high psi and 190 C, Zn Hcl and chlorobenzil, alkaline hydrolysis of desylthioglycolic acid, benzyl
MgCl + benzamide, Acetyl benzoin and mineral acid.
which of these or others would be most realistic for this project?
dimethyamine: methylamine + formaldehyde. it appears that some of the rather otc reactions for methylamine can be adjusted to higher temps and higher
concentrations of formaldehyde to produce dimethylamine.
so what am i missing? (besides sleep and the clear head needed to write something like this, oh yes and the ability to spell anything correctley) any
other ideas? can anyone find the info on the seperation of the different isomers? i cant find any data on them.
if there is a hurdle that maybe a little to hard to jump in this synthesis that can be overcome by changing the end product into something similar
with equal or greater value... point it out. 
i understand that open discussion of these sort of compounds are looked down upon here. for that reason i really would like to keep this thread on
topic and ask everyone to leave out any and all BS.
thanks
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kafka  |
...a theoretical synthesis to this compound from easily available chemicals. not for illicit purposes, simply because of the chalange
and enjoyment of reaching the theoroetical goal.
...anything producing a reasonable amount will be adiquete.
...if there is a hurdle that maybe a little to hard to jump in this synthesis that can be overcome by changing the end product into something similar
with equal or greater value... |
Let me see if I get this right... You want us to help you come up with a synthesis of an illicit compound, produced in reasonable quantity, using OTC
materials, and if thats not possible you want something similar of equal or greater value? I'm skeptical...
Not only that, you appear to have a lack of chemical knowledge, and seeing as your "theoretical synthesis" could entail some toxic chemicals (e.g.
Mercury and/or its compounds) then I would be worried that your "theoretical product" might end up killing your "theoretical customers"... But what
the hell do I know...
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | a paper discussing the original synthesis has not been found by me |
Have you just not looked? They are for
sure out there. Oh, I see: you just want to be spoonfed a recipe.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Let me offer a few suggestions, because I don't really care what you want this compound for (it's probably nothing magnificent anyhow). I'd rather you
learn some chemistry.
<b>1.)</b> Friedel-crafts reactions are a textbook favorite, and otherwise a pretty big pain in the ass (unless you're working with highly
active subtrates or intramolecular reaction). So scrap that idea. You simply won't get a clean enough product, and if you're fixated on OTC then you
probably don't have the means to purify liquid products.
<b>2.)</b> I urge you to move beyond Shulgin. The Al/Hg reduction is neat, but there are excellent reductive amination reactions which
are anhydrous and will not hydrolyse the imine back to the ketone. Please look into NaBH4 reduction of pre-formed imines, and also hydrogenation of
imines formed <i>in situ</i>.
<b>3.)</b> Dimethylamine + formaldehyde... no. You need to learn about oxidation states. If an alkane is considered to be in the "zero"
oxidation state and an alcohol considered "+1", then formaldehyde and any other aldehyde is "+2" (carboxylic acids +3, ureas +4) then the reaction of
methylamine (0) with formaldehyde (+2) must undergo a net reduction to get to dimethylamine (0). <b>R</b>eduction <b>I</b>s
<b>G</b>ain (of electrons).
<b>4.)</b> Stop researching chemistry in patents. Patents are garbage and poorly descriptive (i.e. difficult to learn from). What you
need to do is buy some elementary and intermediate organic chemistry books, and ready <b>primary</b> literature (scientific journals) for
procedures.
An excellent elementary textbook
An excellent intermediate textbook
<b>5.)</b> Which of your proposed routes would be realistic? None. Here's how to do it easily. Grignard addition of benzylmagnesium
bromide to benzaldehyde. Oxidation of the resultant alcohol to the ketone (pick your favorite oxidation.. PDC or PCC is damn easy. bleach is
possible). This gives you "deoxybenzoin" (if want to sound naive) or 2-phenylacetophenone (if you want to sound like a chemist). If you don't know
how to name compounds, draw the structure in the search engine at Sigma Aldrich (Here). Reductive amination with dimethylammonium chloride in a buffered reaction or formation of the imine from dimethylammonium acetate and
subsequent reduction with NaBH4 will yield racemic product
<b>6.)</b> Reductive amination will give a pair of <b>enantiomers</b>. Knowledge is respectable, so try to learn the lingo.
It's important because then you'll recognize that enantiomers have identical physical properties. You might, however, be able to separate these
enantiomers with a fractional crystallization (lactic or tartaric acid to form a <b>diastereomeric</b> salt).
P.S. Another possible route - condensation of benzaldehyde with dimethylammonium salt to give the iminium ion followed by reaction with benzyl
grignard. 2 steps to racemic product.
P.P.S. Chemistry gets a lot more fun if you move beyond the fixation with OTC. There are plenty of safe ways to buy chemicals, and they're perfectly
lawful. My main concern is that you store them properly at your 'lab' (house), so please ask if you need help.
[Edited on 17-1-2010 by Arrhenius]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You are missing the point of this forum, which is not to spoonfeed those who are so lazy as to open a thread without providing one single reference
where needed and then arrogantly asking others for information about making some drug that might even be illegal where you live. You even go as far as
saying that you found no paper on the synthesis of lefetamine, which makes it obvious to everyone that you never ever ventured into a library. Most
members here would even pass over your petty criminal intents if you at least show a decent interest in chemistry and some minimum effort, but your
discourse shows anything but that.
The simplest synthesis of lefetamine is also what is probably its first synthesis. Made by Stevens using the Stevens rearrangement. I will make just
as much effort as you did in providing any reference - that is none.
| Quote: | | i understand that open discussion of these sort of compounds are looked down upon here. for that reason i really would like to keep this thread on
topic and ask everyone to leave out any and all BS. |
You should have done so in the first place by using a scientific discourse. Now that you miserably failed in doing so you invited others to continue
at the same BS level.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | | <b>3.)</b> Dimethylamine + formaldehyde... no. You need to learn about oxidation states. If an alkane is considered to be in the "zero"
oxidation state and an alcohol considered "+1", then formaldehyde and any other aldehyde is "+2" (carboxylic acids +3, ureas +4) then the reaction of
methylamine (0) with formaldehyde (+2) must undergo a net reduction to get to dimethylamine (0). <b>R</b>eduction <b>I</b>s
<b>G</b>ain (of electrons). |
I think he was talking about making dimethylamine via the well known (and annoyingly low yielding) NH4Cl + CH2O route.
Quote: Originally posted by Arrhenius  | | P.P.S. Chemistry gets a lot more fun if you move beyond the fixation with OTC. There are plenty of safe ways to buy chemicals, and they're perfectly
lawful. My main concern is that you store them properly at your 'lab' (house), so please ask if you need help. |
Oh yes, "desoxybenzoin" is probably not any more suspicious than the various precursor to either desoxybenzoin or the Stevens rearrangement (very
interesting - never heard of that!) reactant.
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
How about
1) benzoin + hydroxylamine -> oxime
2) reduce the oxime to the amine
3) methylate the amine to the tertiary amine using paraformaldehyde and oxalic acid (with microwave treatment) or use the Eschweiler-Clarke reaction
4) reduce the OH group using I2 and H2S (with plenty of ventilation for the H2S), or you can use red phosphorus in place of H2S if its available.
According to some things I found in Google books, H2S can be made by heating sulfur and a hydrocarbon such as mineral oil or paraffin wax.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Arrhenius: FC-reactions work fine under reasonable conditions. So long as you have a site that is almost certain to react in your ring (i.e.
stereoelectronically favoured) then FC-reactions are fine. Acylation is obviously superior if you want mono-substitution but alkylation can work
reasonably well in some cases. Reduction of the phenyl ketone that results is relatively easy, and I believe it can be effected by the use of H2/Pd
under acidic conditions (e.g. in glacial acetic acid). Sodium borohydride also works in acidic conditions, as do a few/many other reagents.
You've completely lost me on point 3... It appears you've made a mistake regarding what Kafka actually wrote, but even then what you've
written does not make sense. As I see it, methylamine is on the oxidation level 1.
You also say that reductive amination yields a racemic product. This may indeed be true, but I would at least expect a small enatiomeric excess, even
with boohydride. LiAlH(OtBu)3 would give a much better enatiomeric excess, and from my mental working it looks like it would even favour the
enantiomer given on the Lefetamine wikipedia page (which I am assuming to be the required isomer).
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
turd: Howdy. I don't think anything except methylamine is suspicious here. I don't even think benzaldehyde is (it's OTC anyway). Benzyl bromide?? if
you're THAT concerned (I wish folks wouldn't be) then make it from benzyl alcohol, which is not suspicious at all.
DJF90: yes, +1 on methylamine, pardon me. Please draw me a reaction mechanism for the reaction of formaldehyde and methylamine that does not involve
a two electron reduction. And I'm sorry, but any reductive amination here will give a racemate. Even lithium tri-tBuO aluminum hydride or
L-selectride will give a racemate. There is no thermodynamic or kinetic preference for one face of the imine over the other. Please draw a transition
state that is more thermodynamically favorable for one face if you believe you're correct. An enantiomeric excess *might* be achieved using the CBS
reduction, but I don't know if this works for imines... I found a paper on it, but I'm busy in the lab right now.. will read it later. Unless you've
run a hydrogenation to reduce a benzyl ketone, I wouldn't make the claim that it's facile. Try it.
[Edited on 18-1-2010 by Arrhenius]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by Arrhenius  | | I don't think anything except methylamine is suspicious here. I don't even think benzaldehyde is (it's OTC anyway). |
See number 22
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Nevermind this comment... waste your time being paranoid while I do chemistry.
[Edited on 18-1-2010 by Arrhenius]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Arrhenius: The reason I suggest the lack of a racemate is due to Cram's rules. Draw a newman projection and check it out. Or go read about Cram's
rules. Obviously the larger the nucleophile, the more pronounced the effect will be. And I didnt claim the reduction of the benzyl ketone to be
facile, I said phenyl ketone, which is a different matter altogether and from the literature I've seen, it is facile 
And yes, reductive amination/alkylation using formaldehyde is a reductive process, but that wasn't what my quarm was about.
[Edited on 18-1-2010 by DJF90]
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
mmmm... I only have two of the chemicals from list 1 in stock, but I have nine out of eleven from list two, and I have absolutely no interest in
making illicit drugs. That says more about the lists, I think, than it says about me.
[Edited on 18-1-2010 by Paddywhacker]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | | turd: Howdy. I don't think anything except methylamine is suspicious here. I don't even think benzaldehyde is (it's OTC anyway). Benzyl bromide?? if
you're THAT concerned (I wish folks wouldn't be) then make it from benzyl alcohol, which is not suspicious at all. |
Yes, I should have said just as unsuspicious instead of not any more suspicious. The Stevens rearrangement sounds like a really nice project, just the
pharmacology seems to be bleak... 
| Quote: | | The reason I suggest the lack of a racemate is due to Cram's rules. Draw a newman projection and check it out. Or go read about Cram's rules.
|
I read the wikipedia article and I don't get it. Can you explain to the uninitiated by which magic a symmetric (by mirroring) molecule can give an
enantiomeric excess. Is there some weird diastereomer involved?
| Quote: | | if there is a hurdle that maybe a little to hard to jump in this synthesis that can be overcome by changing the end product into something similar
with equal or greater value... point it out. |
Please define the evaluation function.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Back to main topic.
Synthetic references can be found via Google:
http://www.chemsynthesis.com/base/chemical-structure-6716.ht...
The best starting point for amateur seems to be desoxybenzoin.
It can be prepared from easily available benzoin by simple reduction (without LAH or borohydrides, see references given in OS for desoxybenzoin)
Next: desoxybenzoin-> its oxime -> reduction -> methylation (in this case very good would be formalin/formic acid way) -> racemic shit.
A little cheaper and easier way than suggested by Vogelzang.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | You also say that reductive amination yields a racemic product. This may indeed be true, but I would at least expect a small enatiomeric excess, even
with boohydride. LiAlH(OtBu)3 would give a much better enatiomeric excess, and from my mental working it looks like it would even favour the
enantiomer given on the Lefetamine wikipedia page (which I am assuming to be the required isomer).
|
You can not have asymmetry in a reaction if every component and media is symmetric. An asymmetric induction is only possible where you already have a
chiral centre in the substrate, reagent or catalyst in which case already the transition states are in diastereomeric relationship, thus having
different thermodinamic stability, and consequently different activation energies exist for these transition states (hence the induction). Since in
this specific case none of the possible substrates (BnCOPh or its enamine with Me2NH) have a chiral centre there can be no asymmetry induction unless
a chiral reagent or catalyst with enantimeric excess is used.
The most rational approach would be to use chiral catalysts like in the example of asymmetric reduction of the BnC(=NH)Ph imine described in EP1191030
or in the assymetric alfa-benzylation of the Bn2C=NBn imine (Tetrahedron: Asymmetry, 6, 1225-1228). Chiral auxiliaries can also be used, but
that is not that rational any more (for one example see Synthetic Communications, 19, 1423-1430). The product of all these reductions, one or
the other enantiomer of PhCH2CH(NH2)Ph, needs to be N-dimethylated to give lefetamine or its enantiomer.
An asymmetric reduction of the PhCH=C(NMe2)Ph enamine using chiral catalysts to give lefetamine directly would in my opinion be harder to achieve than
is the case with the asymmetric synthesis of PhCH2CH(NH2)Ph. Though I guess it might be possible somehow.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | You can not have asymmetry in a reaction if every component and media is symmetric. An asymmetric induction is only possible where you already have a
chiral centre in the substrate, reagent or catalyst in which case already the transition states are in diastereomeric relationship, thus having
different thermodinamic stability, and consequently different activation energies exist for these transition states (hence the induction). Since in
this specific case none of the possible substrates (BnCOPh or its enamine with Me2NH) have a chiral centre there can be no asymmetry induction unless
a chiral reagent or catalyst with enantimeric excess is used. |
The words asymmetric and symmetric are very unfortunate in that context and should be replaced by chiral and achiral. For example levo- and
dextrotartaric acid are symmetric by twofold rotation but are definitely chiral. Chirality is the absence of improper rotations (i.e. operations with
det(r)=-1) but says nothing on proper rotations. Same thing for crystals (e.g. quartz which has trigonal symmetry but is chiral). Also "chiral centre"
is problematic, but you know all that of course... 
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
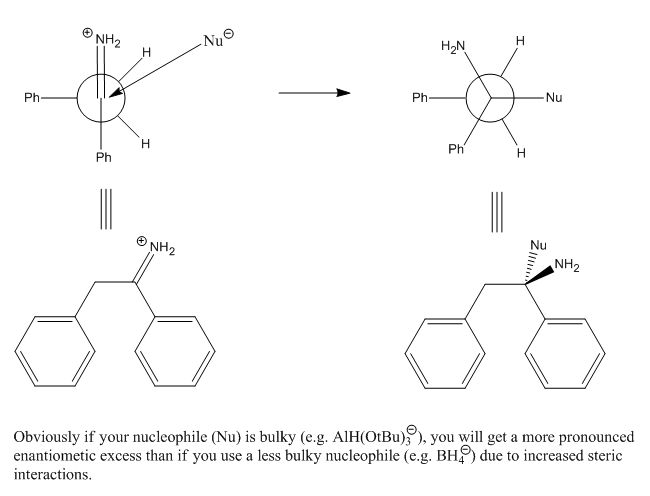
Nicodem: I was not implying asymmetric induction, although Cram's rules covers that, generally by chelation, and this is a method of altering the
stereoselectivity obtained in the reaction. I've attached an image I made quickly in ChemDraw to show what I mean...
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
??
Why is the first picture kinetically or thermodynamically favored over its mirror image?
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Felkin-Anh, Cram & Burgi-Dunitz angle
DJF90: Sorry but Felkin-Anh selectivity would apply in this case, not Cram, which is usually 'anti-Felkin' in the presence of a chelating moiety
(usually beta, sometimes gamma to the carbonyl). That being said, Felkin-Anh will still give no facial selectivity here. The newman projection in the
upper left is the Felkin model, which applies here. Rotate the phenyl (in the back) 180º and you'll get attack over the other benzylic hydrogen,
giving the opposite enantiomer. Cram's rule does not apply here! Use the Felkin-Anh model and you'll find that there's no selectivity.
However, add an R group at the benzylic position (in back on your Newman) and you will direct attack of the nucleophile (hydride) over the hydrogen
and give a <b>pair of enantiomers</b> with <b>anti</b> configuration.
Also! (this is very important!) Your nucleophile is drawn coming in at the wrong trajectory. It should approach at the Burgi-Dunitz angle of 107º
from the "carbonyl".
I've incorporated all of these things in this drawing. Notice that there <b>is</b> a ~5Kcal/mol barrier of rotation about the C-C bond of
the Newman projection, but there is no thermodynamic preference for one rotomer over the other. A barrier of <15Kcal/mole will equilibrate at room
temperature, so it's fair to say we will have both in our reaction mixture.
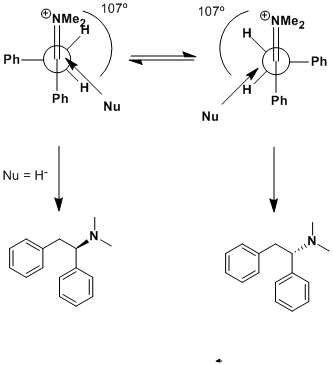
For being dismissed as a "kool" thread.. I'm glad we're discussing some very important concepts.
[Edited on 18-1-2010 by Arrhenius]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Arrhenius: Thanks for pointing this out. It appears that over the past year I've not looked at this stuff enough and have confused it. I recall now
that Cram's rule is generally "the chelate effect", giving anti-selectivity to the Felkin-Ahn model - which appears to be what I was meaning all
along. Burgi-Dunitz I agree is 107 degrees from the carbonyl; my drawing clearly shows what a long time its been since I've used this chemistry.
The point that I didnt think about before I was writing is that "at the back" we have tw hydrogens and a phenyl group, and hence we can get no
selection. This will be the "asymmetric induction" that Nicodem was talking about in his previous post. My apologies for my misunderstanding/lack of
memory.
[Edited on 18-1-2010 by DJF90]
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
http://www.orgsyn.org/orgsyn/prep.asp?prep=CV4P0605
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Fairly high yielding overall, and I'm sure similar yields could be had using the dimethylimine. Alternatively, one could follow that procedure and
then methylate the product. Eschweiler-Clarke conditions are appropriate here, as its not possible to over methylate to the quaternary ammonium salt.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Haha.. cool. I proposed that route just up thread =P
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Paddywhacker  | | I only have two of the chemicals from list 1 in stock, but I have nine out of eleven from list two, and I have absolutely no interest in making
illicit drugs. That says more about the lists, I think, than it says about me. |
Words of sanity! Are "they" going to raid every kitchen? If we reached a point were everything you do means breaching some obscure law, then it's time
to stop paying too much attention to the law.
| Quote: | | I've incorporated all of these things in this drawing. Notice that there is a ~5Kcal/mol barrier of rotation about the C-C bond of the Newman
projection, but there is no thermodynamic preference for one rotomer over the other. A barrier of <15Kcal/mole will equilibrate at room
temperature, so it's fair to say we will have both in our reaction mixture. |
And even if the barrier was orders of magnitude higher the first part of the argument still holds: no thermodynamic and/or kinetic preference for any
of the enantiomorphous rotamers so you would get them in the same ratio. (Unless you invent a novel prion like autocatalytic reaction and manage to
somehow induce an enantiomeric excess to start with.)
The first step is intriguing. I would have thought that the methylamine would be removed with the water phase. Does this work for phenylacetones...?

|
|
|
| Pages:
1
2
3 |