| Pages:
1
2 |
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Far more powerful than Astrolite
N-dimethyl hydroxylamine nitroformate is far more powerful than hydrazinium nitrate, while also being much more resistant to shock.
By the way, pentaborane mixed in carbon tetrachloride is explosive. Normally CCl4 is rather inert and is used in some fire extinguishers.
Another compound of purely academic interest was BH3NH2NH3+ClO4-. It is an adduct of BH3 on hydrazinium perchlorate. BH3 cannot be used itself as it
is too reactive. Rather the adduct of ethyl ether and BH3 introduces the BH3 to the hydrazine. This energetic compound is extremely powerful, and
explodes with a green flash and a lot of dense white smoke. It is rather sensitive however.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: |
N-dimethyl hydroxylamine nitroformate is far more powerful than hydrazinium nitrate, while also being much more resistant to shock.
|
Where did you get this information? From a brief google search I can see that there is very little information on this interesting explosive. It's
really too bad that both n,n-dimethylhydroxylamine and nitroform are such a pain to synthesize... Sadly negates much of its usefulness..
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Over the years, researchers have made many little improvements to "astrolite". First they mixed in some dimethyl hydrazine to replace a fraction of
the hydrazine. Then they replaced the nitrate with a perchlorate, and later dinitramide. There is a paper online that describes symetric dimethyl
hydrazinium nitroformate as very resistant to shock, with potential military applications. Hydrazinium nitroformate is being tested as an experimental
rocket fuel, having a specific impulse almost as good as NH4ClO4, but having no poisonous HCl exhaust fumes. There is a whole site about this if a
google search is done. That it can be used as an oxidizer in a rocket demonstrates the high stability of the nitroformate ion. Obviously hydrazinium
perchlorate is a sensitive explosive.
Hydroxylamine is even better than hydrazine, it contains its own oxygen. Consider this, hydrazine is not an explosive compound, whereas NH2OH is.
Dimethyl hydrazine has a higher specific impulse than hydrazine, meaning oxidation of the methyl groups is more energetic than amine groups.
I believe that bromoform CHBr3 and AgNO2 dissolved in benzene will react to give a low yield (12%) of nitroform (HC(NO2)3) which is acidic. Bromoform
can be made from Br2, NaOH and acetone or ethyl alcohal, similar to chloroform.
Bromines can come off carbon in some situations, whereas methyl chloride will not react with AgNO2. Ag+ is soluble in benzene (or toluene), see
AgClO4, that is how CH3ClO4 is made. For example methyl iodide reacts with AgClO4, in benzene solvent, to make CH3ClO4. I think H2O solvent would not
work. Also, of interest, AgF reacts with iodine to make AgI precipitate and IF5. IF5 is not a strong oxidizer, but hydrolyzes with water to HIO3 and
HF. IF5 could perhaps be used to make pure SbF5 from SbF3. You may see the topic "Advanced SuperAcid Chemistry" if you are interested in Lewis acids
Also, NH2OH can be made from nitromethane and 20% HCl quite easily, see my post about trinitropropane. NH3(OH)Cl probably reacts with CH2O
formaldehyde (used to preserve biologic specimens) to form CH3NH2(OH)Cl and you might also get some dimethyl-hydroxylamine salt because actually CH2O
and NH4Cl will form some (CH3)2NH2Cl depending on conditions when they are boiled. There is an excellent site, http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/m...
[Edited on 16-6-2010 by Anders Hoveland]
[Edited on 16-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Also, something I invented:
dinitro pyridine reacts with liquid nitrosyl perchlorate NO+ClO4- to form an adduct. The ON+ sticks onto the nitrogen in the ring, transfering its
positive charge, sort of a Lewis acid-base neutralization. The formula for the new compound-salt is C5H3N4ClO9. It is much more insensitive to shock
than NOClO4, and would be expected to be extremely powerful. I have only used plain pyridine so far. By the way, NOClO4 is quite soluble in
nitromethane, you can see liquid explosive mixtures go off on youtube. NO2ClO4 is far more of a reactive oxidizer and will readily nitrate other
compounds. It should not be mixed with nitromethane, as toxic volatile C(NO2)4 would result.
NO+ is formed when NO and NO2 are together bubbled into concentrated H2SO4.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | Also, NH2OH can be made from nitromethane and 20% HCl quite easily, see my post about trinitropropane. NH3(OH)Cl probably reacts with CH2O
formaldehyde (used to preserve biologic specimens) to form CH3NH2(OH)Cl and you might also get some dimethyl-hydroxylamine salt because actually CH2O
and NH4Cl will form some (CH3)2NH2Cl depending on conditions when they are boiled. |
I'll admit you have some very interesting ideas. But I'm not convinced all of it is based in reality.. You seem to be doing a lot of guessing with
your reaction schemes.. Hydroxylamine and formaldehyde do not react to form (di)methylhydroxylamine in any literature I have found. It would only make
formaldoxime, or possibly some amino acids..
To get dimethylhydroxylamine you have to oxidize dimethylamine with H2O2 + tungstate catalyst.. And the yields seem to be inevitably very low with
much byproducts formed.. Not to mention the problem of synthesizing and purifying the dimethylamine to begin with... Not what I would call practical.
Sure the synthesis could be accomplished, but I don't see how it could be much more than a novelty explosive.. sadly.
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
The chemistry of explosives has been well understood for decades. Furthermore the universities and big labs have had enormous resources to throw at
the problem. So most everything that is usefull has aleady been patented and/or put to use. Here "usefull" cosiders many factors, power, stability
cost etc. For each of those compounds that have been put to use, many others have been evaluated and forgotten.
A home experimenter may develop something that is interesting and "useful" in the context of home experimentation, ie can be made using available
materials but its almost impossible to invent something better than what is allready in use.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | . . . its almost impossible to invent something better than what is allready in use. |
That almost sounds defeatist. . .
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
"Formaldoxime" CH2NOH probably does not exist. CH2O reacts very differently from acetone. Formaldoxime would probably be the trimer, an RDX with OH
instead of NO2 groups. CH2O reacts with NH4OH to form hexamine for example. I bet the "formaldoxime" trimer reacts similarly to hexamine when HCl
solution is added.
I would disagree that everything has been well researched in academic institutions. They have become increasingly underfunded. In some areas of
chemistry, there are only a handful of knowledgeable experts. I have found few papers about hydroxylamine energetic materials, for example, nothing
about hydroxylamine nitroformate. 1,2,2- trinitropropane has not previously been described.
I am glad my "ideas" are found by someone to be "very interesting". No, they are not all based in reality, otherwise what I could design would be very
limited, however I have a think most of my ideas have a fair chance of working. And although I have a very extensive knowledge of very esoteric
reactions, there are many gaps in my understanding of basic chemistry. I have so many facts in my mind, but I cannot always remember the references/
sources.
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gregxy  | The chemistry of explosives has been well understood for decades. Furthermore the universities and big labs have had enormous resources to throw at
the problem. So most everything that is usefull has aleady been patented and/or put to use. Here "usefull" cosiders many factors, power, stability
cost etc. For each of those compounds that have been put to use, many others have been evaluated and forgotten.
A home experimenter may develop something that is interesting and "useful" in the context of home experimentation, ie can be made using available
materials but its almost impossible to invent something better than what is allready in use. |
Forsooth!
US use of industrial explosives in 2008 was 3,420 thousand
metric tons. Developing a better i.e., less expensive blasting
agent would be a license to print money. A lot of companies
are at work on it.
Often forgotten by the great unwashed masses on la Net —
explosives are tools. Users of explosives like mechanics
use whatever tool does the job for least cost.
Even really expensive Detcord is used in (really pretty) rock
mining because it does the best job. (Reference avail on request.)
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | "Formaldoxime" CH2NOH probably does not exist. CH2O reacts very differently from acetone. Formaldoxime would probably be the trimer, an RDX with OH
instead of NO2 groups. |
Yes you're right it may exist as a trimer.. or a monomer but, trimer or monomer, my point is the same: you can't methylate hydroxylamine with formaldehyde... Although it may raise some
interesting possibilities of using formaldoxime trimer to make some other energetics..
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
A pessimist see's the difficulty in every opportunity
An optimist see's the opportunity in every difficulty
- Winston Churchill
Half a century of dedicated investigation has brought the art as well as science
of energetic materials research to where it is now confidently affirmed that the
reasons why those explosives which are known to work well , is understood.
The most important contribution to our understanding of the detonation process has been
the discovery that the performance of an explosive is a very sensitive function of the number
of detonation products. Systems with high heats of explosion yield detonation products that
have large molecular weights and hence low specific number of particles , so the extra energy
is present primarily as thermal energy rather than intermolecular repulsion.
Nitroguanidine has a heat of detonation half that of Composition B , but it has the same
performance because of the favorable particle population of detonation products resulting
from the high hydrogen content in the explosive and consequent water content in the
detonation products.
Traditionally organic explosives have relied on populating carbon skeletons with nitro
groups. The natural progression has ended in compounds comprised of nothing else ,
such as Hexanitrobenzene. Practical considerations of stability and reactivity compromise
many otherwise promising candidates. Of late High nitrogen content heterocyclic
compounds are regarded with interest. Forum member Engager often demonstrates
this is an area that is not beyond what an able chemist can produce.
Forum member Mendeleev outlined research approaches for improved energetics.
http://www.sciencemadness.org/talk/viewthread.php?tid=1177#p...
PHILOU Zrealone outlined the range of selection of explosophores which may be applied
http://www.sciencemadness.org/talk/viewthread.php?tid=1778#p...
Generation of explosophores ( Handy chart form )
http://docs.s.u-tokyo.ac.jp/pub/%E5%AD%A6%E5%A4%96/Kanri/kan...
Government sponsored investigation into speculative energetic materials.
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
My own foray assessing a hypothetical High Energy Density Material
http://www.sciencemadness.org/talk/viewthread.php?tid=12452#...
A monumental effort of applied research already done is related in this book
Ignition - History of Liquid Rocket Propellants
http://library.sciencemadness.org/library/books/ignition.pdf
Some of the actual results are detailed in the following reports
* N O T E : Titles repeat for the following items but each is a different report
Explosives Research
http://handle.dtic.mil/100.2/ADA303551
http://handle.dtic.mil/100.2/AD025036
http://handle.dtic.mil/100.2/AD006572
http://handle.dtic.mil/100.2/AD021247
http://handle.dtic.mil/100.2/AD006187
http://handle.dtic.mil/100.2/AD006188
http://handle.dtic.mil/100.2/AD009986
Explosives Group Report
http://www.osti.gov/bridge/servlets/purl/459856-APvtjU/webvi...
Research In Nitropolymers and their Application to Soild Smokeless Propellants
http://handle.dtic.mil/100.2/AD006681
http://handle.dtic.mil/100.2/AD009985
http://handle.dtic.mil/100.2/AD035617
http://handle.dtic.mil/100.2/AD010068
http://handle.dtic.mil/100.2/AD007557
http://handle.dtic.mil/100.2/AD000988
.
[Edited on 18-6-2010 by franklyn]
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
For routine blasting it seem like it would be hard to beat
something like tovex or other NH4NO3 based explosives
for low cost and safety. See for eg 3664897, Just AN, Al
and water and claimed to be cap sensitive down to 35mm.
There are already many patents on these going back 50 years.
(although I guess the drugies could get the methylamine out of
Tovex to make meth).
TNT is probably the best aromatic, PETN the best nitric ester, or EGDN if you want liquid,
HMX or RDX the best nitroamine.
The are all simple compounds which function "well enough" and can be made from
readily available materials (in an industrial setting).
1,2,2Trinitropropane may be interesting, but I can't see much advantage over
nitromethane (unless it is a solid) and it would be more expensive to make.
Primaries may be the best area to research since the old favorites have heavy
metals and are now considered bad. Plus making an effective, stable safe primary
is much more of a balancing act, making it worth the effort to create a more complicated molecule. But, I would think the big labs could analyze the
most
interesting candidates in a week inside a computer.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I have a whole thick binder full of ideas, both easy ways to make extreme starting chemicals, and new molecular structures.
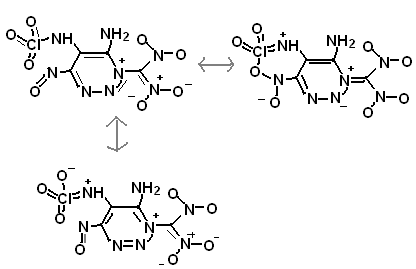
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
The compounds in the above post might look highly unstable and sensitive, but notice the delocalized electrons. Just as perchlorate and nitroformate
are stable because an extra electron resonates on the oxygen atoms, so to would that whole compound.
[Edited on 20-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
To get dimethylhydroxylamine you have to oxidize dimethylamine with H2O2 + tungstate catalyst.. And the yields seem to be inevitably very low with
much byproducts formed[/rquote]
I wonder, would NaOCl bleach mixed with dimethylamine make some dimethylhydroxylamine? Or would you only get tetramethyl hydrazine? If the latter,
perhaps it would form a diperchlorate that would be insoluble in water, since those methyl groups would make it more hydrophobic.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  | | Quote: | | "Formaldoxime" CH2NOH probably does not exist. CH2O reacts very differently from acetone. Formaldoxime would probably be the trimer, an RDX with OH
instead of NO2 groups. |
Yes you're right it may exist as a trimer.. or a monomer but, trimer or monomer, my point is the same: you can't methylate hydroxylamine with formaldehyde... Although it may raise some
interesting possibilities of using formaldoxime trimer to make some other energetics.. |
Actually CH2=NOH has an equilibrium with CH3NO, which can be reduced with bisulfite to make CH3NHOH. So you can at least put one methyl group on
hydroxylamine. Then, reacting this with more CH2O, it would probably condese to
CH3N(OH)CH2N(OH)CH3, Upon addition of HClO4 solution in water, this would likely disproportionate into
mono-methyl hydroxylamine perchlorate,
N,N di-methyl hydroxylamine perchlorate, and formic acid.
However, if it is really true that "you can't methylate hydroxylamine with formaldehyde" then I am unsure what products would form.
"A small amount of hydrazine blended in nitromethane can increase the power output even further. With nitromethane, hydrazine forms an explosive salt"
(wikipedia)
This salt would likely form a powerful combination with hydrazinium perchlorate. The hydrazine salt
NH2NH3(+) H2C=N(O)--O(-) could be mixed with hydrazine perchlorate to form a super powerful astrolite variation.
It is doubtful that
ClO4(-) (+)NH3NH3(+) H2C=N(O)--O(-) could exist, since the nitro methane would probably separate out as oily liquid since the salt likely only forms
at higher pH.
CH3NO2 has been added to NH4NO3 instead of fuel oil to give a more powerful explosive. CH3NO2 would also make hydrazinium perchlorate more energetic,
but the reaction rate would be limited by the small-scale separation of the hydrazinium perchlorate crystals and the oily nitromethane, no matter how
well the dispersion was mixed.
Making nitromethane in anionic form would allow it to completely mix with the oxidizer, dramatically increasing the rate of reaction of the NH2NH3ClO4
providing oxygen to completely burn off the CH3NO2. Possibly a solid fusion mixture would prevent the two compounds from crystalizing separately. Such
a fusion could be carried out at low temperature since the melting points for the two compounds would be expected to be quite low. Perchlorate and
nitromethyl anions might even form a double salt with hydrazinium cations.
|
|
|
br25
Harmless

Posts: 3
Registered: 1-7-2010
Member Is Offline
Mood: No Mood
|
|
nitromethan with 80%+ H2O2 ist very powerfull and NM with TNM.Its possible to mix NM with ammonium persulfate or isopropyl nitrate?
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Nitromethane is not ideal since it has a low density. The salt of nitromethane, however, would be expected to be much more dense since ionic
intermolecular forces are involved. At only 10 g/100 mL in water, I would not expect CH3NO2 to be much more soluble in H2O2. I think nitromethane
could only dissolve up to 10% H2O2. This would make the CH3NO2 only somewhat more powerful. I am unsure if, when liquid A is known not to be very
soluble in liquid B, that means that liquid B is not going to be very soluble in liquid A ? Using only 80% H2O2 would further diminish the benefit of
the improved oxygen balance of the mixture. Addition of surfactant would allow the two liquids to mix in more ideal ratios, but on a microscopic
scale, the nitromethane would still be separated out as little oily droplets suspended in the H2O2. This would have a significant detrimental effect
of the det. velocity, but the energetics and heaving power would not be affected. It should also be realized that the surfactant would take up around
4% of the volume, perhaps it would be simply burned off by the H2O2 and not be a complete waste.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Silly question... But anyone know the process to freebase my Hydroxylamine Hydrochloride? I have forgotten.
I may try refluxing it with an excess of formaldehyde... If that is what is needed in theory to form this 'N-dimethyl hydroxylamine'.
Nitroform is within reach. This looks possible...
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I'm pretty sure you'll be wasting your time. The formaldoxime formed will immediately be tied up in it's trimer form and not react further. If it was
that easy industry definitely wouldn't screw around with oxidizing dimethylamine or pyrolyzing tertiary amine oxides to make dimethylhydroxylamine in
lowish yields..
There are several other ways you can make it though..
Try making some dimethylamine from hexamine + HCl, freebasing it, adding sodium tungstate (pretty easy to get) at 1-5% of the weight of the
dimethylamine to an aqueous (or possibly alcoholic, etc.) solution of the dimethylamine, then drip in an equimolar amount of H2O2 solution over an
hour or so.. Not sure what the best way is to isolate it. Maybe it forms an insoluble salt with something? Or form the sulfate, chloride, etc and try
to salt it out with a water miscible solvent like acetone.. The yields probably won't be great but it will work.
Or if you can somehow make/acquire N,N-dimethylcyclohexylmethylamine you could do it via this route. Good luck..
Or you could try the reaction of ethyl nitrate with a methylmagnesium halide.. Haven't found many details on this reaction.. You could probably
clarify it with some more research. Ethyl nitrate is a bit of a pain to make, so I don't know how useful this is..
[Edited on 9-7-2010 by 497]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  | I'm pretty sure you'll be wasting your time. The formaldoxime formed will immediately be tied up in it's trimer form and not react further.
Or if you can somehow make/acquire N,N-dimethylcyclohexylmethylamine you could do it via this route. Good luck..
[Edited on 9-7-2010 by 497] |
I do not think that the trimer would be unreactive. Consider that hexamine reacts with HCl to give the the HCl salt of methylamine and formic acid.
(Keep in mind that CH2=NOH has a tautomer in equilibrium of CH3NO )
I think the reaction described could be performed with triethylamine instead. Or dimethylamine could react with acetic anhydride, giving AcN(Me)2,
which can be reduced to
EtN(Me)2. "HI reduces certain α-substituted ketones and alcohols replacing the α substituent with a hydrogen atom." (wikipedia). I also
found mention that HI is "used to reduce carbonyl groups" on a site by "Rhodium". It can be clearly seen that a R3C--CO--CR3 is reduced to
R3C--CH2--CR3.
Investigation of the Impurities Found in
Methamphetamine Synthesized from Pseudoephedrine
by Reduction with Hydriodic Acid and Red Phosphorus
K.L. Windahl, M.J. McTigue, J.R. Pearson, S.J. Pratt, J.E. Rowe, E.M. Sear, Forensic Science International, 76, 97-114 (1995)
EtN(Me)2 reacting with H2O2 and heated to 160C, would presumably make ethylene and (Me)2NOH.
Organic Syntheses Prep is an excellent site, containing all sorts of surprising reactions, some of which can actually be useful for energetic
compounds.
Anyway, you need not get too caught up in having to make an exact molecular replicate when you hear about ideal properties, you can often improvise.
For example, the perchlorate of EtNHOH would probably be just as powerful, though not as insensitive.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Another thought
HOCH2CH=O <--> O=CHCH2OH
2-hydroxyl ethyl aldehyde should condense with hydroxylamine to form
O=N--CH2CH2--N(OH)--CH2CH2--N=O
There are two optional routes from here. It can be oxidized with H2O2 to HON(CH2CH2NO2)2, or NO2 can be bubbled in to make HON(CH2C[NO2]3) through the
oxime tautomer. Let me again remind of the potential for powerful, but highly stable energetic salts with the first of these compounds.
The above is somewhat speculation. Although H2O2 will apparently oxidize trimethylamine, I think dimethyl hydroxylamine would be more resistant.
I am going to include the procedure in the link above, just in case it becomes unavailable in the future. The picture is included.
In a carefully cleaned 500-ml. Erlenmeyer flask, covered with a watch glass, are placed 49.4 g. (0.35 mole) of N,N-dimethylcyclohexylmethylamine ,
39.5 g. (0.35 mole) of 30% hydrogen peroxide, and 45 ml. of methanol. The homogeneous solution is allowed to stand at room temperature for 36 hours.
After 2 and 5 hours hydrogen peroxide (39.5-g. portions each time) is added, The excess hydrogen peroxide is destroyed by stirring the mixture with a
small amount of platinum black (Note 4) until the evolution of oxygen ceases. The solution is filtered into a 500-ml. round-bottomed flask and
concentrated at a bath temperature of 50–60°, a water aspirator being used initially and an oil pump finally, until the amine oxide hydrate
solidifies. A Teflon-covered stirring bar is introduced into the flask, which is then connected by a 20-cm. column to a trap (reversed to avoid
plugging) cooled in Dry Ice-acetone. The flask is heated in an oil bath to 90–100°, and the apparatus is evacuated to a pressure of ca. 10 mm. with
stirring of the liquefied amine oxide hydrate. When the content of the flask resolidifies, the temperature of the oil bath is raised to 160°. The
amine oxide decomposes completely within about 2 hours at this temperature. Water (100 ml.) is added to the contents of the trap. The olefin layer is
removed with a pipette and washed with two 5-ml. portions of water, two 5-ml. portions of ice-cold 10% hydrochloric acid and one 5-ml. portion of 5%
sodium bicarbonate solution. The olefin is cooled in a Dry Ice-acetone bath and filtered through glass wool (Note 8). Distillation over a small piece
of sodium through a semimicro column2 yields 26.6–29.6 g. (79–88%) of methylenecyclohexane, b.p. 100–102°
The aqueous layer is combined with the two neutral aqueous extracts and acidified by addition of 45 ml. of concentrated hydrochloric acid. The
solution is concentrated under reduced pressure at 60–70° until no more distillate comes over. The residue, which solidifies on cooling, is dried
in a vacuum desiccator over potassium hydroxide pellets to yield 30.7–32.7 g. (90–96%) of crude N,N-dimethylhydroxylamine hydrochloride, m.p.
103–106° (sealed tube). Crystallization from 40 ml. of isopropyl alcohol gives 26.6–30.7 g. (78–90%) of the pure hydrochloride, m.p.
106–108°
HOCH2CH=O might be possible from H2O2 and acetylene
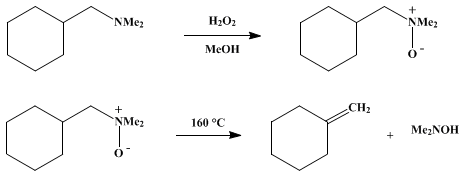
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Anders, silly question... But, where can I go with 200 grams Hydroxylamine Hydrochloride on my bench?
Can I form salts with it? I thought about condensing with an aldehyde to see what happens... Ethanal maybe. Then whatever forms gets 'Massed and
Gassed' in September, and 'perchlorated' (reacted with HClO4) as I call it.
I coulf of course make DNAF or AAAF again but 'been there done that'.
Perhaps I can react it with aminotetrazole and then form some kind of funky high nitrogen complex?
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | 2-hydroxyl ethyl aldehyde should condense with hydroxylamine to form O=N--CH2CH2--N(OH)--CH2CH2--N=O |
I'm a little confused about your proposed mechanism here. I assume you mean the hydroxyacetaldehyde will form an oxime, which will then tautomerize to
nitrosoacetaldehyde, react with more hydroxylamine to make nitrosoacetaldoxime, which then condenses with another nitrosoacetaldehyde to make your
final di(2-nitrosoethyl)hydroxylamine?
I'm not sure if it would go like that though, for one thing, there is literature that says that the nitroso group may stabilize the enol form enough for it to be the more stable than the keto form. This might
prevent it from reacting with further hydroxylamine, I'm not sure.
Also, as with synthesis of dimethyhydroxylamine, I don't think an aldehyde can N-alkylate an oxime, which is what you're saying should happen right?
Do you have a mechanism for this? From what I've seen that scheme can only take place if the oxime is first hydrogenated to a monoalkyl hydroxylamine, which will react with a
further amount of aldehyde.. and then that must be hydrogenated again to get the final dialkyl hydroxylamine. Not quite so easy..
Further casting doubt on your hypothesis, I found a patent where in one example they synthesize hydroxyacetaldoxime using excess hydroxylamine. They
say nothing about it condensing, dimerizing, or whatever. So unless they just didn't notice or the conditions must be different, it seems that
reaction doesn't occur.
Still, hydroxyacetaldehyde and derivatives look very interesting and very novel in the energetics realm from what I can tell. I found a nice looking
route to it using fairly available chemicals too. Basically the idea is chlorinate vinyl acetate (pretty cheap online) in an alcohol solution with Cl2 to make chloroacetaldehyde dialkylacetal, then drip that into a
NH2OH*HCl solution, basify with NaOH, extract with immiscible solvent and voila hydroxyacetaldoxime in supposedly good yields according to the patent. From there maybe react with NO2, etc and get some 2,2-dinitroethyl nitrate?  That would be some crazy stuff, if it was stable.. Many other possibilities to contemplate too. That would be some crazy stuff, if it was stable.. Many other possibilities to contemplate too.
[Edited on 9-7-2010 by 497]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Your idea is actually fairly creative, and yes, I think it would work. However, bubbling NO2 to a ---CH=NOH would likely give ---C(NO)(NO2) because
the hydrogen site becomes vulnerable when the carbon is double bonded to something else. For example CH2=CH2 reacts with chlorine to make,
CH2=CHCl and HCl. If you want ClCH2CH2Cl, you have to do the reaction with heat and pressure. I think that
---C(NO)(NO2) would be easily oxidized to the trinitro with dilute H2O2, but I am really not sure, and if it did work, the compound would probably
become even more sensitive to shock. Nitroso groups tend to be fairly reactive. Anyway, I think (NO2)2CH2CH2ONO2 would only be a little more sensitive
than ethyl nitrate. Isopropyl nitrate is not explosive.
Also, I think you have to do a regular HNO3/H2SO4 nitration to get the nitrate group on. Nitrogen dioxide will not work. You want to do the acidic
nitration AFTER there are no hydrogens on the same carbon atom with the nitro group(s), otherwise you will only get a --COOH, after the hydrolysis,
disproportionation, and oxidation.
HOCH2CH2=NOH --> HOCH=CH--NHOH --> O=CH--CH2NHOH
HONHCH2CH=O and NH2OH --> HONHCH2CH=NOH
HONHCH2CH=O and HONHCH2CH2--NO condense to...
HONHCH2CH(OH)N(OH)CH2CH2NO , which then...
okay, I see the problem. I made a mistake. Two of the intermediate molecule above would condense.
...............................HONCH2CH--N(OH)CH2CH2NO
.........................................l.........l
.ONCH2CH2N(OH)--CHCH2NOH
So let me modify my original idea:
HONHCH2CH2=NOH reacts with Ac2O (acetic anhydride)
to make AcN(OH)CH2CH2=NOH and AcOH
then NO2 is bubbled in, making AcN(OH)CH2C(NO)(NO2),
then conc. NH4OH is added, which hydrolyzes off the acetyl
NH4OH + AcN(OH)CH2R --> NH4OAc + HN(OH)CH2R
so now you have HONHCH2C(NO)(NO2)2 as the final product
Another idea, use ethylene and a mix of NO and NO2 to make NO2CH2CH2NO, then reduce with bisulfite to
NO2CH2CH2NHOH, then make the perchlorate salt, but do not use too much acid or you will hydrolyze the NO2 group.
[Edited on 10-7-2010 by Anders Hoveland]
|
|
|
| Pages:
1
2 |