| Pages:
1
2 |
romanceliu
Harmless

Posts: 16
Registered: 14-9-2008
Member Is Offline
Mood: No Mood
|
|
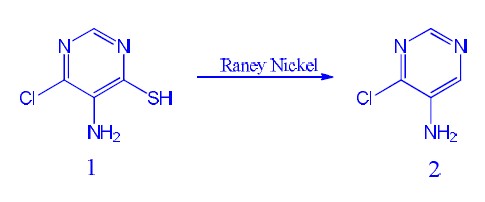
desulfuration
now we synthesis the chemicals
5-AMINO-4-CHLOROPYRIMIDINE from chemcal 1,
process:
1 was added to 3L flask and added NH3.H2O and EtOH was added subsequence. The mixture was stirred and Raney-Nickel was added portions, then stirred
the mixture overnight. the mixture reflux for 2 hours. TLC show reaction completed, cooled to rt and filter to remove solid, concentrated filtrate
and purified by silica-gel got yellow solid
but Raney-Nickel need a lot
1:Raney-Nickel=1:5
i want to find another method to desulfurate , i search a lot of Literatures , but i do not good method except Raney-Nickel
pls gei me some advice , i want to find to good method to
desulfurate
thanks
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Is that the minimum amount of Raney nickel to obtain complete conversion? Does lowering the Ni loading and prolonging reaction time still give
incomplete conversion? What is the ratio of the dehalogenated product? Your post lacks the most basic information.
You might be able to dramatically reduce the amount Raney nickel if you use it as catalyst instead of reagent, but this would require a hydrogenation
apparatus in order to supply external hydrogen. Rate of dehalogenation might increase though. Also, the activity of Raney nickel is highly dependent
on the method of preparation (type of catalyst). Which Raney nickel version do you use? Try another one.
Otherwise, there is a method where aluminium-nickel (50:50, the alloy used to prepare Raney Ni) and the substrate is refluxed in formic acid. This
might require less Al-Ni alloy and avoids the tedious preparation and handling of the pyrophoric Raney nickel. If you truly did a literature search,
you surely already know about this, so I will not bother searching for the reference. Though I do not know whether such method leads to increased
dehalogenation or not.
PS1: Are you aware that this is a forum for amateur chemists and that your industrial profit related question is - besides being non hygienic - also
considered a form of parasitism? If you insist on exploiting us, then at least have the decency to make a forum donation, or at least start
contributing to the community!
PS2: Start using a spell checker!
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
My general protocol for nickel desulfurisations is to reflux the substrate with 2-3 mol eq. raney nickel in acetone or methyl ethyl ketone until TLC
indicates complete conversion. The metal quality required is the oldest and crappiest nickel possible since I do NOT want any hydrogen left in the
pores of the catalyst. To make absolutely sure the catalyst is dead dump it in some acetone and reflux the shit out of it for about thirty minutes.
Then change solvent and start the reaction. I prefer not to use alcohols as solvents in this reaction since they can activate the catalyst again.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
The catalyst needs adsorbed hydrogen, otherwise it is unable to replace the C-S bonds with C-H ones. Raney Nickel is the traditionally used reagent,
but Urushibara Nickel and "Nickel boride" also remove sulfur from organic molecules. Urushibara nickel in particular is worth looking into, as it
could be a much cheaper alternative to Raney Nickel, and is supposedly easier to prepare.
If the Raney Nickel is not freshly prepared (so as to contain adsorbed hydrogen gas), then the reaction must be performed under hydrogen
atmosphere.
|
|
|
Oxydro
Hazard to Others
  
Posts: 152
Registered: 24-5-2004
Location: NS, Canada
Member Is Offline
Mood: distracted
|
|
DJF90, I read the first post, thought, "Hey! U-Ni sounds ideal here"... scroll down and you got here first.
http://www.erowid.org/archive/rhodium/chemistry/urushibara.h...
This pretty much covers it. Note that:
A) catalyst is easy to produce, and quite cheap.
B) is recoverable and easy to regenerate.
C) is suitable (allegedly) for all applications of Raney nickel.
"Our interest's on the dangerous side of things" -- Browning
|
|
|
romanceliu
Harmless

Posts: 16
Registered: 14-9-2008
Member Is Offline
Mood: No Mood
|
|
thanks , i will try ,
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Sorry Oxydro, you just gotta be quicker 
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | You might be able to dramatically reduce the amount Raney nickel if you use it as catalyst instead of reagent, but this would require a hydrogenation
apparatus in order to supply external hydrogen. Rate of dehalogenation might increase though. Also, the activity of Raney nickel is highly dependent
on the method of preparation (type of catalyst). Which Raney nickel version do you use? Try another one.
Otherwise, there is a method where aluminium-nickel (50:50, the alloy used to prepare Raney Ni) and the substrate is refluxed in formic acid. This
might require less Al-Ni alloy and avoids the tedious preparation and handling of the pyrophoric Raney nickel. If you truly did a literature search,
you surely already know about this, so I will not bother searching for the reference. Though I do not know whether such method leads to increased
dehalogenation or not. |
I have never seen Raney nickel being used in hydrodesulurization since the mechanism behind the activity of nickel here is the irreversible formation
of NiS. Can you please give an example of the catalytic use of nickel in hydrodesulfurization?
If using 50:50 Ni-Al with formic acid as hydrogen source, doesn't that give a raney-type of nickel as residue with all the pyrophoric properties as
ordinary raney nickel?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nicodem mentions the formation of the Raney Nickel in situ and so there is no pyrophoric properties to have to deal with (at least until work
up).
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
DJF90, What makes you think that wasn't pretty obvious when I wrote "If using 50:50 Ni-Al with formic acid as hydrogen source"?
It is in the work up the pyrophoric properties becomes a problem.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Of course, but thats 50% less pyrophoricity you have to deal with, if there is none whilst setting up the reaction.
[Edited on 27-6-2010 by DJF90]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Barium  | | I have never seen Raney nickel being used in hydrodesulurization since the mechanism behind the activity of nickel here is the irreversible formation
of NiS. Can you please give an example of the catalytic use of nickel in hydrodesulfurization? |
I was completely wrong here. The nickel catalysts used for catalytic desulfurizations are very different from Raney nickel (Ni, NiS or Ni/Co
molybdates on alumina) and the reaction requires temperatures above 200 °C, so this is something else altogether and not applicable to sensitive
substrates. Like you say, at any lower temperature than that, NiS should always be the end product instead of H2S, so that catalysis or use of less
than 1 eq of nickel is out of question.
| Quote: | | If using 50:50 Ni-Al with formic acid as hydrogen source, doesn't that give a raney-type of nickel as residue with all the pyrophoric properties as
ordinary raney nickel? |
I don't think formic acid is used as the hydrogen source here. It is probably just the acid for etching Ni-Al and forming hydrogen saturated Ni in
situ. I should check the original paper, but I can't remember where I saved it (my collection of files is getting too chaotic to handle). It
might have even been acetic acid and my memory confused them. The residue in my opinion should not be pyrophoric as the conditions are not such to
form the Raney nickel type of material. But that is just an opinion and I'll rather not claim anything this time, because when it comes to this topic
you surely have more experience. I never did any desulfurisations and my experience with Raney nickel is limited to its use in catalytic hydrogenation
of alkenes and nitriles for which I used it only 5 times, of which I managed to get it to burn one time while filtering it of after the hydrogenation.
 Luckily the fire did not spread to any nearby solvent, but the hazards of
Raney nickel should never be underestimated. If I compare that incident with the only similar incident with Pd-C which occurred to me only once in
about 100 hydrogenations it soon becomes obvious that palladium on charcoal is a piece of cake for handling when compared to Raney Ni. Another
incident with Pd-C that I had was the typical consequence of loosening the safety protocols due to lowered fears (one of the most common reasons of
all fuck ups!). Seeing it is (seemingly) no big deal, I soon begun to skip the argon flushing of the vessel before adding 5% Pd-C to the methanolic
solution of the reaction mixture and one time the contact with Pd-C ignited it. Luckily the neck of the vessel is not wide enough and the flames
extinguished by themselves as soon as the air could not sufficiently access the solvent any more, but the experience was scary enough to get me back
to religiously stick to the safer practice. Hope this little account helps others in avoiding similar accidents. Luckily the fire did not spread to any nearby solvent, but the hazards of
Raney nickel should never be underestimated. If I compare that incident with the only similar incident with Pd-C which occurred to me only once in
about 100 hydrogenations it soon becomes obvious that palladium on charcoal is a piece of cake for handling when compared to Raney Ni. Another
incident with Pd-C that I had was the typical consequence of loosening the safety protocols due to lowered fears (one of the most common reasons of
all fuck ups!). Seeing it is (seemingly) no big deal, I soon begun to skip the argon flushing of the vessel before adding 5% Pd-C to the methanolic
solution of the reaction mixture and one time the contact with Pd-C ignited it. Luckily the neck of the vessel is not wide enough and the flames
extinguished by themselves as soon as the air could not sufficiently access the solvent any more, but the experience was scary enough to get me back
to religiously stick to the safer practice. Hope this little account helps others in avoiding similar accidents.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by DJF90  |
If the Raney Nickel is not freshly prepared (so as to contain adsorbed hydrogen gas), then the reaction must be performed under hydrogen
atmosphere. |
That's bollocks, I have 10 years old RaNi containers and it works as good as new. I would never dare to run a reaction with RaNi under hydrogen atm...
However, as long as it's wet it's perfectly safe to both store and handle... I've used it for a number of hydrodeselenizations and sometimes I've been
sloppy so it catches fire (burns a bit like a sparkler, no problem), especially when I used magnet (Nickel is magnetic!) and had to clean it after the
rxn. 
[Edited on 1-7-2010 by Sandmeyer]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Good to know. It is annoying to prepare it every time when needed.
| Quote: | | I would never dare to run a reaction with RaNi under hydrogen atm... However, as long as it's wet it's perfectly safe to both store and handle...
|
It is actually perfectly safe all the way to the filtration step, as long as you don't do anything stupid. What I did was to prepare the W4 version,
but rather than filtering, doing all the washing steps by decantation. This way you can never have it exposed dry and you end up with a thick slurry
of Raney Ni in water. Then you just use a pipette to add it in the reaction vessel (you estimate the amount by volume and mass of the Al-Ni alloy
used). It works beautifully for reducing nitriles. In one case I got complete reduction in matter of 30min, while it took 2 days using Pd-C! It is not
only that Raney Ni is more active on certain functional groups, but also in that you can load as much catalyst as you want (it is dirt cheap!).
Otherwise certain functional groups get reduced with RaNi while they practically don't with Pd-C (ketoximes are one such annoying example).
| Quote: | I've used it for a number of hydrodeselenizations and sometimes I've been sloppy so it catches fire (burns a bit like a sparkler, no problem),
especially when I used magnet (Nickel is magnetic!) and had to clean it after the rxn.  |
It is not the glowing and burning metal that is scary, it is the sparks flying around in the vicinity of highly flammable solvents that get me
extremely nervous. Otherwise, I think the most common source of fires in organic labs is sodium. The metal burning, not the hydrogen explosions! That
was the scariest experience I ever had and about a third of colleagues that I ask about say their worst fire accident was with sodium.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
In your 10 year old containers, do you have 50:50 Ni/Al alloy, or the actual prepared Raney Nickel itself? I was using the term "freshly prepared" to
mean "contains hydrogen", whilst Barium was advocating the use of purposely dehydrogenated Raney Nickel; Something that would not work without a
hydrogen atmosphere for the hydrodesulfurisation of C-S bonds, which is what the OP requests.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by DJF90  | | In your 10 year old containers, do you have 50:50 Ni/Al alloy, or the actual prepared Raney Nickel itself? |
Raney Nickel (RaNi) - as I already said.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Just wanted to be sure; I've seen Raney Nickel advertised before on ebay but it was just the alloy.
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | | Barium was advocating the use of purposely dehydrogenated Raney Nickel; Something that would not work without a hydrogen atmosphere for the
hydrodesulfurisation of C-S bonds, which is what the OP requests. |
Can you please provide a reference in which RaNi is used as a catalyst to convert a C-S bond to C-H and H-S-H?
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
I think he means that RaNi with hydrogen completely removed from its surface is dead and incapable of hydrodesulfurization and I agree. The new
hydrogen atom(s) on the product has to come from somewhere, and it comes from the RaNis surface. I have been unable to get hydrodesulfurization to
work when I have boiled the RaNi in aceton for a while prior to adding it to a complex thiocompound dissolved in various solvents. Without boiling, it
works great, well, too great, as I get other functional group reduced as well and I want it spared. There are many literature examples where they boil
RaNi in various solvents to get it deactivated in order to make it more chemoselective, but I think there still must be at least some hydrogen present
on the surface otherwise it dosen't work.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yes this is exactly what it meant. Was my communication so poor that it took this long to understand?
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by DJF90  | | Yes this is exactly what it meant. Was my communication so poor that it took this long to understand? |
I understood you perfectly, but I also pointed out that you were wrong in saying that one needs freshly prepared RaNi for the reaction to work - no
one in his right mind makes his own RaNi these days. RaNi dosen't have to be "freshly prepared" in order to contain hydrogen as you imply, mine is 10
years old and contains hydrogen, as I already mentioned. 
[Edited on 2-7-2010 by Sandmeyer]
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
What happens with the old hydrogen atom(s) then?
In the example given by the originator of the thread a C-S-H functionality is to be converted to C-H and Ni-S, a reaction which doesn't need any
additional hydrogen as it is only a removal of the sulfur.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Sorry, I was just assuming the OP didn't have access to Raney Nickel, and thus has to prepare it from the alloy. "Freshly prepared" was used in the
wrong context; I'll be more careful in future.
Good point Barium. I forgot we were cosidering a thiol and not a sulfide. Its an interesting point, but I'm not sure it will work without additional
hydrogen. I know this is slightly off topic, but thiols will react with gold surfaces in order to adhere the molecule to the surface via the sulfur
atom. Nickel may react similarly, but I susppect you are correct in this instance.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by Barium  |
What happens with the old hydrogen atom(s) then?
In the example given by the originator of the thread a C-S-H functionality is to be converted to C-H and Ni-S, a reaction which doesn't need any
additional hydrogen as it is only a removal of the sulfur.
|
Yes, I agree with you, there is enough hydrogen atoms (equal amount in reactants as well as products) in the case of thiols. The hydrogen atom on that
C-S-H is just acidic and AFAIK you can't treat dead RaNi with an acid to make it active - it has to be hydrogen gas, which is absorbed onto the
surface of nickel by providing one electron per hydrogen atom to the d-orbital (where the juicy valence electrons are) of nickel. So for example, I
think that attempted hydrodesulfurization with dead RaNi conducted in acetic acid would fail. However, I should also add that I'm just guessing. 
[Edited on 2-7-2010 by Sandmeyer]
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
I don't think I have ever seen a desulfurization using Raney nickel using hydrogen from external sources. It has alvays been a intramolecular
donation. If not, then why isn't nickel used in catalytical amounts? I believe the reason for using nickel in the form of Raney nickel is due to the
very high surface area compared to other forms of nickel. Since the end product is NiS and not H2S, high loadings of metal is needed in order to
provide enough nickel to bind all sulfur.
I'd still love to see a reference in which nickel is used as a catalyst in a desulfurization or hydrodesulfurization.
|
|
|
| Pages:
1
2 |