Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
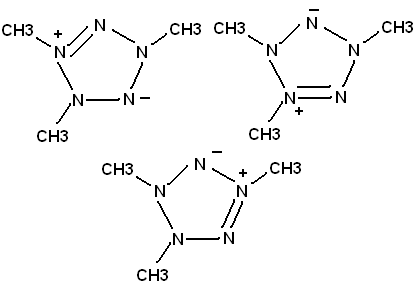
1,2,4-trimethyl pentazole
I reacted CH3NHNO with methylamine, and near anhydrous Mg(ClO4)2. Silver acetate is just a little bit soluble in a mixture of the final solid product
and lighter fluid, so I suspect I may have made the pentazole. CH3NHNO has equilibrium with CH3NNOH
Putting nitro groups on the methyls would make a good energetic compound. My "pentazole" is not very unstable; this may reflect the declocalized
bonding in the nitrogen ring. It will, however make a pop noise when hit by a hammer.
I realize that in all likelihood, I probably made something other than a pentazole. But I think
CH3NNN(CH3)NNCH3 will spontaneously cyclize to the ring.
I might also try monomethyl hydrazine chloride and
CH3NH3+(NO2-), which might form a pentazole in low yield, but only have dimethylN2H2, which would not work.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
If I'm right CH3-NH-N=O leads to methanol...via a very unstable hydroxydiazonium...
it is known from ages that alkanic amines doesn't form stable diazoniums upon reaction with HNO2....and rapidly turns (if water is present) into
alcohol and various derived oxydation products...
CH3-NH-N=O <==> CH3-N=N-OH --> CH3° + N#N + °OH --> CH3-OH + N2(g)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Reference
Anders Hoveland

Posts: 13
Registered: 12-7-2010
Member Is Offline
Mood: No Mood
|
|
Phenyl pentazole, and other substituted phenyl compounds with a pentazole group have been made in the lab for some time now. The stability of
phenylpentazole along with para-substituted and ortho,para-substituted arylpentazoles, The stabilizing effect of the electron-donating groups is shown
to be due to a resonance interaction with the electron-withdrawing pentazole ring.
The Stability of Arylpentazoles , by Peter Carlqvist, Henrik Östmark, and Tore Brinck
J. Physical Chem 2004
I was unable to find any [cyclic] pentazoles with more than one group attached, but one would think that additional groups would only add stability.
(1-(5-tetrazolyl)-4-guanyl tetrazene hydrate) is prepared by reacting sodium nitrite with an aminoguanidine salt dissolved in acetic acid at 30–40
°C. This is the most relevant thing I found. Reference: Field Guide to Clandestine Laboratory Identification and Investigation, By Donnell Christian
There do not seem to be any primary nitrosamines, R-NH-NO , besides intermediate radicals, that come up in a search. While, this group is probably
very unstable, I am uncertain that the mechanism proposed by PHILOU Zrealone is correct. Azomethane does not seem to spontaneously decompose into
radicals. The Preparation of Azomethane, by Francis P. Jahn
J. Am. Chem. Soc., 1937, 59 (9), pp 1761–1762
September 1937,
Azoxymethane, which is said to be the oxide of Azomethane. Azomethane (CH3-N=N-CH3) should not be confused with Diazomethane, which is a rare example
of a stable radical, others being nitrogen dioxide.
Ab initio calculations relevant to the mechanism of chemical carcinogenesis, by N-nitrosamines. Rosanna Bonaccorsi
|
|
|
|