| Pages:
1
2
3
4 |
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
If the window is visible to passers by, it might be better to use a thick sheet of clear Plexiglas (PMMA) over the window and attach the blower to
that. That's what I've done and it's never attracted any attention from the curious folk who seem to be everywhere. Even better to also plant a
shrub in front of the window. Even better if you have no neighbors, but a low profile is always good.
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by entropy51  | | If the window is visible to passers by, it might be better to use a thick sheet of clear Plexiglas (PMMA) over the window and attach the blower to
that. That's what I've done and it's never attracted any attention from the curious folk who seem to be everywhere. Even better to also plant a
shrub in front of the window. Even better if you have no neighbors, but a low profile is always good. |
Don't worry, my duct will be covered by a bunch of bushes and would look like any other laundry dryer duct if someone saw it.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Are you filtering the output?
If not, the legality of what you're making aside, those that be may become upset should someone ever notice the odd smells and their skin tingling as
they pass by; if they can get close to the vent. Some of the foul smelling things I've produced have released copious clouds of persistent fumes for
hours, that people can smell a long, long way away. Even after being blown out, they'll hang in the air like smoke from a fire; causing people to call
the authorities out thinking your house is on fire.
High up on the roof is the best place for it, where it'll be diluted and washed away by the higher air stream. There is a pocket of much slower moving
air around ground level.
Failing that, having it vent to the back garden side of the house will give it time to spread out a little more.
It's lazy, and dangerous, to be letting the harmful fumes escape the glass in the first place. Morally, I think it's a bit of a shitty thing to be
then sucking the fumes away from yourself and spraying them towards someone else. Particularly if there are kids around.
Activated carbon filters are supplied in a vast array of sizes and capacities by the hydroponic stores selling to cannabis growers. They work
exceptionally well for a lot of odors. I've run them and actually had the place smell fresher than it did before I started producing things that
stink.
Sorry if this is redundant and you will be filtering. I just don't see the point of going on about safety and then risking someone else's when it
involves hammering together a big hood, all this work and when the filter isn't much more.
All thar best,
John
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
My only option is to vent at ground level (with a bunch of bushes in front), but I'll follow your advice and neutralize as many fumes as I can before
they can leave the glass, and work on a small scale. And I can always work on windy days if needed.
If it still stinks outside, I'll consider adding a filter as you suggested. How much resistance do those carbon filters add? Do you think my blower
can push the air through one and still have enough face velocity?
[EDIT] Maybe I could just blow the fumes through a good filter and release them back into my lab. Then I wouldn't have to worry about getting
the duct through the window and fumes stinking up the whole neighborhood etc. etc. AND having a source of air to be sucked into the hood wouldn't be a
problem. How many gasses do those filters stop? I'd need them to stop: Cl2, NO2, SO2, Br (small amounts) small amount of acid fumes from conc.
solutions, burning fumes like burning Mg or steel wool, etc. etc. I'm not going to be doing much organic chem, so whatever scary fumes are created
through O-Chem are not a problem. What do you think about a ductless hood?
@peach: Thanks for the suggestions, you're not being redundant.
BTW, I've heard of "gas wash heads", but what are they? How are they different than gas washing bottles like this?
[Edited on 5-8-2010 by bob800]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
If you're the kind of odd ball person I find floating around a number of these forums, you could also run the work once it's gone 11 or 12 at night,
when most people will be home and probably in bed. I'm quite often up at 4am messing around. Watching people walking up and down the road hitting
things with a garden spade, for example. 
If you blown enough corrosive or dirty shit out the exhaust, that bush in front of it may begin to take on a chemically toasted appearance. If the
rest of the garden is vibrant green, the bush may make the exhaust stand out even better to passers by than the exit it's self, when they hear the fan
noise and notice a bush near were it's coming from has gone brown.
You may wish to add a one way flap to the outlet (like a piece of rubber sheeting), such that a gust of wind isn't going to blow the fumes back
through the hood.
Running it on wet or rainy days will also knock a lot of things out of the air. HCl(g) will fall out quickly, for example. You can easily implement a
method to force a constant rainy day on the output by simply sticking a tiny fogger or misting nozzle towards the end of the outlet.
In the UK, we have a brand called Hozelok that sell a ton of cute little micro-irrigation things at the garden centres. They sell a number of nozzles
for producing a cone or fan of mist for the plants. All you need do is get one of those and hook it up to a tap, then turn it on when something nasty
starts coming off. The water usage rate will be significantly lower than it would for an aspirator in the sink. If you're using PVC, corrosion won't
be an issue and you can simply drill a hole for the nozzle to poke in and silicon or epoxy it in place, or a few of them at various angles down the
length to get a good scrub action on the go.
I think I actually have some of those nozzles in the garage. If I can find them, I'll post you one or two for free.
This is a method used in some commercial fume hoods. To deviate a little for a history lesson, in 'ye olde days' they didn't even use a fan. They'd
have a flame at the back of the hood to draw the fumes out by convection. This also happens if the exit is high up, it's called the chimney effect and
it's partly why chemical plants and power stations use chimneys, the air rushing over the top creates a venturi like effect and sucks gas up
automatically. The helical swirls you see on some are vortex breakers, so the airflow around the top doesn't place too much bending load on the stack.
U-Boats used this trick in water when they realized in WW2 that raising the periscope effectively draws a long white line of foam, created by
turbulence, towards the exact location of the sub, for a plane overhead to line up with and then bomb.  Hoffman also commented that he was using flame aspirated hoods in his lab when he produced LSD, when discussing how
basic their equipment was back then despite dealing with something so powerful. For biologists in particular, the flame has the additional benefit of
killing anything organic trying to escape. State of the art aerospace design also makes use of vortex properties. Those little fins you see sticking
vertically out of the wings on an airliner are vortex catchers. By stabilizing the vortex under the wing, it creates high lift efficiency. If they're
allowed to burst under the wing, the pressure difference drops. And the randomized popping creates turbulence. The same effect is now being proposed
for wind turbines, to increase their efficiency. And, yet again, it's also seen in nature. The bumps on the fins of big whales have evolved to be
there as they allow the fin to cut through the water better by controlling the vortexes flowing over it, and so, the turbulence. Hoffman also commented that he was using flame aspirated hoods in his lab when he produced LSD, when discussing how
basic their equipment was back then despite dealing with something so powerful. For biologists in particular, the flame has the additional benefit of
killing anything organic trying to escape. State of the art aerospace design also makes use of vortex properties. Those little fins you see sticking
vertically out of the wings on an airliner are vortex catchers. By stabilizing the vortex under the wing, it creates high lift efficiency. If they're
allowed to burst under the wing, the pressure difference drops. And the randomized popping creates turbulence. The same effect is now being proposed
for wind turbines, to increase their efficiency. And, yet again, it's also seen in nature. The bumps on the fins of big whales have evolved to be
there as they allow the fin to cut through the water better by controlling the vortexes flowing over it, and so, the turbulence.
The activated filters are also standard for modern commercial hoods, the recirculating hoods are largely just a carbon filter. Which worries me
somewhat given the variability of things that'll go through them, as anyone who's looked at respirators will know one cartridge will not safely absorb
every fume. E.g. ammonia and monoxide will pass through carbon filters okay.
I don't know about the precise velocities and such that you'll achieve with the filter in place, but you can buy huge ones and you
can always modify the fan selection. If commercial hoods use them, you'll be able to do it, it's just a matter of cost. Anything another human has
designed or built, you can understand or replicate given enough effort.
Another option would be to use a HEPA filter with an activated carbon back, which will also allow a lot of gas through. You could even fit the filter
behind the baffles, such that most of the nasty shit is absorbed within the hood before it gets to the duct. Big, high flow rate, low pressure drop
HEPA filters will probably work out even cheaper than the hydroponics scrubbers, as they are manufactured in vast quantities, globally and are very
basic in terms of construction, but also effective enough for use in silicon wafer labs, hospitals and biological laboratories. I've never taken apart
a commercial recirculating hood. But I wouldn't be surprised to find a HEPA filter in a number of them.
You're looking for active carbon HEPAs, and the flat kind for the lowest price. You only need the filter element (which are available as consumables
for replacement when the originals run off their ratings or start to clog, after about a year to three of heavy use). To drop the back pressure and
improve the flow rates even more, you could then pull off the micronic filter stage and just have the activated carbon. Taking care not to pull off
whatever is being used to keep it in place, or you'll look like a cartoon character that's just had a bomb go off in their face when you turn it on
(the carbon is incredibly fine dust and black, so there'll be something inside containing it).
All the premade filters aside, you could even produce your own, spend even less and likely have it work even better than a commercial option. I expect
it'd take less than an hour to put together, probably just a few minutes, as the parts are designed to fit together in seconds with no tools.
To do so, I'd;
1.) Buy a bag of activated carbon powder.
2.) Try to find out what it's rough particle size is
3.) Find something with a mesh rating that's slightly smaller than that (like woven cloth or wire mesh); some places sell wire mesh so finely woven it
will filter micronic moisture droplets out of the air, and they often sell it in various metals (like stainless) and in little 1 foot swatches.
4.) Get some PVC sewer pipe and fittings.
5.) Cut a length of it and stick a swatch of material over it, then press the fitting on
6.) Pour in the carbon
7.) Repeat stage 5 for the other end.
That requires zero input from someone who needs the capital to set up a factory and machines, pay peoples wages and make money on it. It's also balls
easy. If you make the carbon section a bit bigger than the charge you add, the particles will also get some opportunity to move around, decreasing the
build up of concentrated layers. It'll also mean the filter has less opportunity to pack and produce back pressure.
In terms of the mesh, the wire will distort the least (messing with the pore rating) as you jam it in place. But more active metals may also rot open,
so a plastic may be better if you're not getting stainless. The pore size should be a little below the dust particle size, but not a lot more. You
just want to trap the dust, making it any smaller will make it more expensive and produce more back pressure, making the fan more expensive too.
The American Society for Testing and Materials (ASTM) counts activated carbon as powered (PAC) when the mesh goes above 80, or below about 0.17mm for
us newbs who can't visualize mesh numbers. This is the same stuff used in the expensive commercial filters.
The stainless mesh is expensive. A 100 micron (0.1) teflon coated mesh is about £20 per square foot. 100 mesh copper (won't react with HCl(g)) is
about £10. Aluminium is around £10. Bronze is £6.25. Brass is about £5. Using a plastic or fabric mesh will be even cheaper.
The prices are from here, but search around and you'll quickly find others doing them by the square foot and they may have discounts on the meshes
you're after in stainless
Have a look at this bit as well, where they discuss the different weave patterns. Dutch is the toughest. Sintered won't distort but costs a lot
more.
It's likely that you'll want to use the largest particle size available (or even a small grain size) if you pack a good length of the pipe, as it'll
be a layer much thicker than most filters so the super fine dust will produce more back pressure. Using larger granulated material will also make the
mesh to retain it even cheaper. I would recommend against packing something like the following access fitting full of fine power; enough to create a
layer will do.
Anywhere from 6 months to years later, depending on what goes through it and how often, it'll need recharging. Pulling apart standard 110mm fittings
is hard work or essentially impossible. You'd need to pay a little more for ones with collets that unscrew. A far, far, far, farrrrr easier option is
to contruct your carbon chamber from a 'drain access', 'drain entry', 'rodding' port or 'access pipe'. That's a short length of pipe with an
unscrewable port on the side. Do the same with it as above, but now when it comes to recharging you can simply unscrew the port, pour out or hoover up
the carbon, pour some fresh stuff in and go.
You can also buy sewer pipe in 160mm, but it's for commercial uses (so less likely to be found in regular hardware stores) and is more expensive (for
obvious reasons). There is an added bonus to using what is called 'vent soil pipe' (the black or grey stuff for above terra firma usage), in that you
can make it look like a normal foul drain even if it's sticking out the side of the house by simply running it to ground level (although I've heard
you crazies in the US run the foul pipes inside the walls sometimes, they're all outside here because all the walls are solid brickwork, making it
hard to do the wall trick). Also, have you considered hooking the hood to the actual sewer? That way there's zero chance anyone is going to connect
the smell or noise with your house. Especially so if the nearest grid in the street isn't directly outside your drive (as it is for me).
In the UK, the foul drain that leads out to the main pipe in the road is connected to it, by law, via a water trap, to prevent
potentially dangerous pathogens and such flowing back up the pipe from material rotting in the public sewerage system. We don't put the traps there,
they're put in by the council when they lay the main sewers and hook the houses up in the road. There is also a 'vent pipe' which runs all the way up
the side of the house to the roof, where any nasty smells or germs can wash away and air can get in to keep the pressures in the pipe correct for the
traps to function.
Hence, if I was to blow a hood's exhaust down my sewer, it'd blow out two stories up where no one would smell it, feel it on them or notice the noise.
Even better, if the vent pipe is near the chimney, to a passing glance any persistent fuming is likely to written off as the result of a log or coal
fire venting where it's supposed to. With the exit being high up, you stand a better chance of benefiting from a natural aspiration, chimney effect
too. Ever seen the water in your toilet being sucked back when it's windy out? That's the aspiration effect, producing quite a fair bit of vacuum in
the pipe (the effect doesn't work very much in reverse, blowing things back down it). And you have somewhere for the water from your misting nozzles
to drain to. Win, win, win eh? Bar the possible need for a stronger fan (or you could get used to keeping the slash closed over, and keeping in mind
that people on here go on about using baffles and adjustable fans to purposefully turn their hoods down when the sash is part
closed). Someone has already built a roof mounted, water trapped exhaust for yar. Yars just need to be connectin' to her.
To pick up on the baffling point, I'm not sure how important it is or if people know about it, but baffling a number of fans causes the blades to
begin turbulating and stalling and the flow numbers aren't the same as simply turning down the juice into it; the rotor isn't slowing, it's slipping
through the air rather than catching it, like a cavitating propeller. Centrifugal fans are prone to this effect. When it starts happening, the flow
rate doesn't fall in a predictable fashion, it vertically drops off. As was the case for the U2 spy plane, who's wings would snap off if it sped up
too much or stall if it slowed down too much at it's 70 - 80k feet cruise altitude; the margin for error in the speed was 10 knots!  This is called the 'death corner' of a planes aerodynamic performance. Airliners
suffer a similar problem, but not as severely. Providing another example where an electronically controlled fan speed will probably be a safer bet
than doing it mechanically, as the stalling won't occur when you turn the drive down. Probably why a number of modern flow hoods and benches use
electronic methods over mechanical baffling, despite it being more time consuming and costly to design, pass through CE testing, produce and fit than
a sliding bit of metal. This is called the 'death corner' of a planes aerodynamic performance. Airliners
suffer a similar problem, but not as severely. Providing another example where an electronically controlled fan speed will probably be a safer bet
than doing it mechanically, as the stalling won't occur when you turn the drive down. Probably why a number of modern flow hoods and benches use
electronic methods over mechanical baffling, despite it being more time consuming and costly to design, pass through CE testing, produce and fit than
a sliding bit of metal.
A 'vented soil pipe' 'access pipe' (£8.94 from toolstation.com in the UK, free next day delivery on orders of £10, free hot drinks in the store
& the lowest prices available, sweeet);

There are also other handy bits available. Such as prebent angle bends (which are helpfully made with a slight off 90 degree angle to encourage
draining) or this, an adjustable bend to take up errors;

Flexible couplings;

These ones are cheaper and come in lots of different configurations to couple from 110mm to various other sizes. They produce the same water / air
tight seal and have big hoze clips to get them tight, as you can see (use a ring spanner or socket set for ease, screwdrivers are a pain in the ass).

There a many more, and the way you set the up is largely limited by your inventiveness.
What you've linked to is essentially a wash head. If you've seen someone going on about them on this forum, there's a good chance it was in one of my
posts, as I rant on about their virtues all the time.
The only difference between mine and the one you've shown is that mine is all glass, because they're also used for analytical, experimental work that
may interact with the rubbers and such in that one. E.g. HCl(g) will slowly rot orange natural rubber and DCM will make it swell up. But you can get
inventive with that two and line the stoppers with PTFE tape or use silicone or poly stoppers.
If you're just using it for scrubbing, I expect that one will do you fine. Along with a number of variations on it that people have made with jars.
Fritted glass is better, but the glass frits also have a tendancy to fall apart in strong base solutions, and the aquarium ones can melt in some
solvents or corrode around things like HCl(g).
I would hazard a guess that the majority of nasty things you'll produce will be either going acidic or basic when in solution and likely soluble in
water; HCl(g) is very soluble, for instance. To scrub HCl(g), you could take that bottle you linked to and simply fill it with extremely concentrated
KOH. With no frits to dissolve, it'll sit there happy as piggies in shit (which aren't actually that happy in actual shit) until you're too old to do
chemistry anymore. Any HCl(g) that attempts to get through it will immediately be raped, hard, to potassium salt. Use NaOH and you've got table salt.
There will essentially zero HCl(g) at the output.
So, selecting to have a frit or not and the material it's composed of depends on the wash in progress. In some cases, plastic will work better than
glass. In others, the plastic will be a molten blob on contact.
You can also stack a number in series to scrub a lot of different possibilities, or increase the efficiency again.
I'll include a picture of the wash heads & bottles QuickFit here in the UK do at the end. This one is a sintered version.
All thar best!
John
{edit}I just noticed the price on that bottle you linked to. YOWZERS! That's a good 10 to 15 times cheaper than the bottle alone for
this one, used.

[Edited on 6-8-2010 by peach]
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
First of all, thanks for writing all that info!
So you're saying I could use something like this: http://www.heavenfresh.com/Replacement-hepa-filter-for-XJ-38.... Or would something like this be better: http://cgi.ebay.com/4-x-12-ACTIVATED-CARBON-CHARCOAL-FILTER-...
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  |
{edit}I just noticed the price on that bottle you linked to. YOWZERS! That's a good 10 to 15 times cheaper than the bottle alone for
this one, used. |
Yeah, I know elemental scientific is REALLY cheap. They have 500 ml ACS Nitric acid for $9.99!!! Only problem is that they're REALLY slow at filing
orders.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
The cylinder will likely be easier to fit into the system.
The HEPA is likely to have finer powdered material in it, whereas that cylinder has granules (I think mine had powder, not sure).
As to which is better for your specific design, that's really something you'll need to have an investigate into, such as checking the recommended
fans, CFMs and pressures of those or other alternatives and thinking about how your want to lay it out or vent it. You can make rough comparisons by
looking at references like 3M, who make a lot of the HEPAs and list their flow characteristics. Filters of a similar size from different companies
will have roughly similar figures attached. The same applies for the cylindrical filter, there will be people selling them who list the flow rates at
different pressures. Asking the stoners at the cannabis growing forums would be a good place to start, as soon as they wake up and stop watching
daytime TV, eating packs of biscuits.
The micronic filtering nature of the HEPAs makes me suspect the flow rate of that granule only cylinder will be higher if they have roughly the same
surface area. You can work that out from the diameter and compare it to the flat sheet filter.
[Edited on 6-8-2010 by peach]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I'd still consider the PVC pipe option. With the pipe, you'll know what all the seals and such are so you'll better know their corrosion properties.
PVC is pretty good in terms of that. The mild steels will start corroding if things like HCl(g) go through. Some of the others components may as well.
I suspect granules will do if you fill most of the access pipe. If you can still smell anything, add a handful of PAC.
Unless you've dealt with things like HCl(g), you'll probably underestimate it's ability to rot things. As an example, I can have all my tapers either
greased or sealed with PTFE tape and keck clipped. If a few tiny puffs of it escape whilst I'm swapping something around, or if the grease isn't well
seated or runs out, things five to ten metres away will start going. My cooker hood is about that far away from the surface I produced some HCl(g) on
a few weeks ago. Despite the amounts of gas escaping being so low they are barely detectable, there was rust appearing on the stainless a week or two
later. Similarly, if I clean the glass out over the kitchen sink, there will inevitably be a few traces of something left and it'll fume a little. Not
enough to be dangerous, but the fumes have rotted a brand new stainless sink to look like it's a decade old, despite me blowing them away and
thoroughly rinsing down the surfaces. In fact! I just remembered I have something I can show you...
This is a pair of stainless steel scissors I had lying on the bench beside me when a small, virtually undetectable amount of HCl(g) escaped the glass,
they were shiny prior to this;

Note how the opposite side is significantly less corroded, indicating just how sensitive things like this are to the flow rates and directions (it's
only stained the top, where it could land, like the germs in Pasteur's broth flasks [Pasteur actually 'stole' a lot of the limelight for germ theory
from the earlier Semmelwies, who was told he was nuts for thinking tiny animalcules were everywhere and he died at a young age in a psychiatric
hospital, after publishing reams of papers regarding their probable existence, his evidence based control strategies and the beneficial results this
achieved in hospitals]);

Despite them having stainless stamped on them, that term is strongly bastardized now to mean anything with any chrome, moly etc content in it that
exceeds mild steel grades, so a lot of the 'stainless' you find around the house isn't actually all that stainless. You need graded, controlled
stainless like 316, Inconel and the others. But it's a fair indicator of how aggressively things like this will attack metals.
In a lot of cases with chemistry, building your own is usually going to end up costing more in money and time, performing unacceptably or being a
risk. I think the access pipe scrubber is reasonable unique in that it is likely to be cheaper, quicker, work just as well or better and present well
known and controllable variables.
John
[Edited on 6-8-2010 by peach]
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
Do you think my blower will corrode too then?
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
If the blades are mild steel and HCl(g) comes into contact with them, yes.
There's a good chance they'll look like those scissors after it touches them the first time.
But with iron, the corrosion can actually help retard further corrosion provided the layer isn't being mechanically wiped or washed off. Out in the
wild, all the rain and things moving around will tend to rub the layer off, which then reforms, gets rubbed off, et cetera until the surface is eaten
through. In your fan, hopefully, the air flow alone won't be enough to do any significant rubbing. You may open it and discover the blades are brown,
but still perfectly intact.
People who paint things commercially purposefully spray all their iron and things with that universal red paint prior to the colour layers. That's not
only a primer / mask, it's red iron oxide and there as a corrosion inhibitor; the poor man's galvanizing.
It's also a bit like cutting metal with a flame torch, plasma cutter or laser. The heat won't really cut through it alone, the molten blob needs
blowing through with gas; mechanical agitation.
Same is true etching PCB's, they want bubble tanks or pumps in the tank to mechanically replace the diffusion gradients and rub the metal coming off
away.
Something with PVC blades would probably come out looking cleaner, but as I say above, a bit of surface muck isn't so bad.
The bearings will almost certainly be stainless steel, and probably closed, so they shouldn't be too much an issue.
[EDIT]I thought of a cheap alternative, or one that'll save you the effort of looking for another fan and doing all the curves again anyway, just
paint the blades with something like enamel. If you're bothered about the appearance of things and the seals, again, you'll want to use 110mm or
bigger soil pipe, the mild steel will rot (not through, but it'll look shitty). Any slight fails in the seams may get the steel going brown on the
outside as well; it only takes a tiny, tiny amount. I can't imagine you'll get a comparable seal on the steel without siliconing it all. Which can
also be messy and come apart with movement. Those 110mm seals take seconds and are easily tight enough for gas at such low pressures (I needed big
hydraulics to pull one open). You can even wrap a layer of PTFE plumbing tape over the OD of the male pipe and it's ends first for a pretty much
chemically invulnerable seal.
[Edited on 8-8-2010 by peach]
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
 . .
On the grainger page, (http://www.grainger.com/Grainger/wwg/search.shtml?searchQuer...) it says the "Housing Material" is rolled steel and the "Housing Finish" is gray
enamel. But I don't think that's referring to the blades. Can you tell what they're made of from the picture?
The enamel paint idea sounds good, but I don't want to paint the blades shut!
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
That's excellent that it's already painted with enamel, you're saving some spray painting there.
I would be very impressed if you managed to spray the blades shut, it'll be next to impossible. I guess you may have been thinking of using a brush
perhaps?
Nah, don't bother. They sell enamel in spray cans at the hardware and car places. It's often called 'radiator paint'.
Take the cover off the fan and try to get the blades and the motor separate from the housing. It'll be super, duper easy to paint something like that,
even easier than most things. Just give the fan a spin (with a stick, not the socket) and spray as it turns. You may want to undo the set screw and
take the blades off entirely to do the back as well. Remember to keep the can 12" or so away, give it a good 2 minute shake first, put on a damp coat
and add more, carefully as per the instructions. If you put your greasy mits all over the blades and don't follow the time intervals on the pack, it
will mess with the paint. Remember there are 'hidden spots' as well, you need to be ducking and leaning all over it under a bright light to find where
you've missed. Spraying outside usually wastes a lot of paint.
I can't really tell what the metal is from that picture, it's bright, but it's also brand new. Stainless is quite a bit more expensive to buy and
process, so it's more likely it's mild steel. They may have galvanized it, but I'd have to have it in my hands to see that ideally. If it's zinc
galvanized, a number of paints won't stick to that without a special primer, so get the fan first or hold onto your spray can receipt.
[off topic]I used to work in a metal fabricators. For one job, they hot dipped a bunch of supports for a railing fence at an airport. Then bought the
special acid etch primer. Then were told by the spray guys one drum of the expensive commercial stuff they'd bought would do the entire job with most
of it left over. They had about ten on the van. Ask first, buy later. 
E.g. Email them and ask if someone can try sticking a magnet to the blades.
[Edited on 8-8-2010 by peach]
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
OK, I'll go with the epoxy idea. Thanks for all your suggestions.
Attached is my "final" plans for the hood, along with the dimensions.
Also, the depth doesn't have anything to do with how many cfms I need, right? The only thing that matters is the face velocity,
right? How deep do you think I should make it?
[slightly off topic]. You said you're using a 1L RBF, right? I don't think I need that much capacity though. Do you think this 19/22 setup would fit
in my 2.5' sq hood:
[url=http://cgi.ebay.com/New-19-22-Chemistry-Laboratory-Distilling-Kit-FREE-S-H-/200383943175?pt=LH_DefaultDomain_0#ht_3129wt_1137]
Again, thanks for all your help.
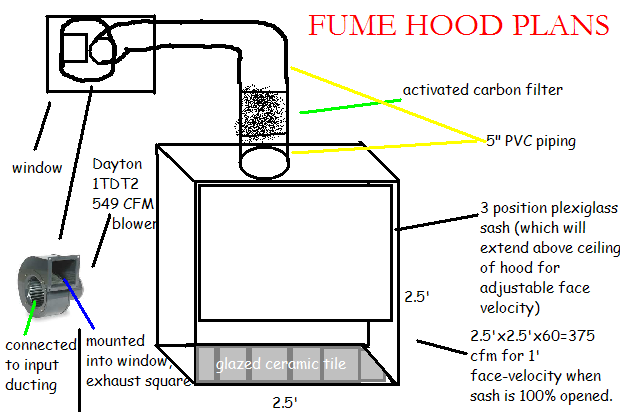
|
|
|
anotheronebitesthedust
Hazard to Others
  
Posts: 189
Registered: 24-6-2007
Member Is Offline
Mood: No Mood
|
|
Do they make blowers with ptfe or other non-corrosive, highly abuse resistant materials without charging $1000's?
I found this site but they are located in China and I have a hard time trusting asians. And I still wasn't able to find a worthy model.
http://www.blower.com.tw/en_pdc00.htm
|
|
|
anotheronebitesthedust
Hazard to Others
  
Posts: 189
Registered: 24-6-2007
Member Is Offline
Mood: No Mood
|
|
Well found what I was looking for from Grainger, but as I thouth the price would be in the $1000's.

http://www.grainger.com/Grainger/PLASTEC-Blower-3MPW3?Pid=se...
But one benefit is that the noise level is only 43 dB
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
You can forget that matey! 
PTFE = big cash even for objects that can be easily made from standard solids without excess machining or special moulding methods. A centrifugal fan
blade is reasonably complex by comparison and theres basically no demand outside of very odd chemical handling problems, meaning they can charge
whatever they like, leaving you with you're anus stinging.
I think it might be worth modifying your attitude towards Asians a little there. I get your point, that it's easy to get ripped off from those
distances and be able to do nothing about it. However, I have ordered quite a few items from China and actually had the guy send it quicker, cheaper,
better and with less hassle than people in my highly developed home town of England.
Also remembering, China is a highly mechanized, technology leading country (a ton of the big name US / European countries have plants out there, not
at home). If you pay for rubbish, they'll give you rubbish. If you pay a decent amount, they can produce equally good products, or better (given that
'they' work a lot harder most of the time). As you can see from the photo, I am not 'one of them'. 
Besides, if you scrub the gas stream a little prior to it hitting the fan, as per Bob's suggestion in his 3 year old looking paint diagram  (jk), most of the corrosives / solvents should be massively diluted down before
they get to the blades. (jk), most of the corrosives / solvents should be massively diluted down before
they get to the blades.
@bob800
>>>>A video reply!<<<<
[Edited on 10-8-2010 by peach]
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
Wow, thank you so much for making that! . I guess it makes up for your MS paint
insult! . I guess it makes up for your MS paint
insult!
[EDIT] I just bought the wood, plexiglass, and blower for the hood. It's going to be 2.5' sq with 2' depth. Hopefully that'll fit a 19/22 setup...
[Edited on 10-8-2010 by bob800]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Glad you liked it. Pictures are a lot more useful most of the time. I'll be stalking you for photos of said fume hood. 
[Edited on 10-8-2010 by peach]
|
|
|
cnidocyte
Hazard to Others
  
Posts: 214
Registered: 7-7-2010
Member Is Offline
Mood: No Mood
|
|
I nearly have my fume hood built, just the hard part left. The fan. One of the gypsum walls of my hood faces the garden so I could literally just
install a box fan right into the wall of the hood but I'll have to cover the motor to make sure it doesn't ignite any flammable fumes. I'm definitely
gonna use solvents like ether and acetone in my hood. I have ADHD and even on dexedrine the main fume hood thread is painful to read so I don't think
theres any harm in having more than one DIY fume hood thread going.
I built the floor of my hood with these old boards I had lying around, I can't remember what the name of this material is but whatever it is it
literally as no chemical resistance, I tested a spare bit of board with some NaOH solution and a day later the insides of it were oozing out. I need
to cover this crap in something chemical resistant. I put down some polypropylene mats (they sell them for putting in drawers).
[Edited on 11-8-2010 by cnidocyte]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I have to bring out my negative nancy hat here and say, that's a bit of a poor excuse. If you can't read the thread, it kind of suggests you might not
be too careful using things that require the hood in the first place.
Acetone isn't much of a fire problem. I smoke and play with my lighter sat right in front of it, with it all over the glass (as I rinse it), the
surface, the paper I'm writing on, tissues, rags, me... I've never seen it ignite, ever, and I've gone through tens of litres of it.
Ether is another issue altogether.
That's where I'd start considering the benefits of a sparkless motor.
With the doors and windows open as I work with misbehaving solvents, the escaping vapour is rapidly diluted by air passing through. In a hood, it'll
puddle up and all have to go out past the fan.
The only time I've had a fire was with cyclohexane. Again, I'd been sat smoking with it all over the place all night. Then someone else came into the
room, tried to light a candle and the piece of paper a few drops had landed on immediately burst into flames; within 30s of them coming into the room.
Indicating that it's not so much the ignition source, as who's controlling it.
I'm clinically insane (quite literally, according to the NHS). They've had me on their special person pills, checking in for hour long chats with
psychiatric teams every two weeks for a year and I only mention it because you mentioned ADHD. I can still read the threads and think they should be
merged. 
[Edited on 11-8-2010 by peach]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by peach  |
Acetone isn't much of a fire problem. I smoke and play with my lighter sat right in front of it, with it all over the glass (as I rinse it), the
surface, the paper I'm writing on, tissues, rags, me... I've never seen it ignite, ever, and I've gone through tens of litres of it. .....
The only time I've had a fire was with cyclohexane. Again, I'd been sat smoking with it all over the place all night. Then someone else came into the
room, tried to light a candle and the piece of paper a few drops had landed on immediately burst into flames; within 30s of them coming into the room.
Indicating that it's not so much the ignition source, as who's controlling it. ........
I'm clinically insane (quite literally, according to the NHS). |
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
L@@K @T>>>>>Yet again, he pounces on the chance to have a complain and ...'s out the reasoning to shape it to how he likes it. This seems to be a very common,
annoying, theme for yourself. You'll actively seek out things to have a complain about when people try to make the offer bit of help (I've seen you
doing it in other people's thread as well, over very minute details), you'll tell me how to word my replies, then you'll actively edit them to fit
your own opinion. The people around you in the real world must be extremely patient. Sometimes, you need someone else to point these things out to
you. Try making your own videos, and you'll see how you appear to the rest of us.<<<<<<I makes you a special video
reply, 'dude'!
[Edited on 11-8-2010 by peach]
|
|
|
cnidocyte
Hazard to Others
  
Posts: 214
Registered: 7-7-2010
Member Is Offline
Mood: No Mood
|
|
I read the thread, I just said it was painful. I'm clinically autistic according to the psychiatrists but you can't believe everything they tell you.
I'm a bit less meticulous with the fan part of my hood because I could literally take a wall off it and use 1 of those portable oscillating fans to
get rid of the fumes.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Unless you're speaking to a consult, who actually has a degree, you're probably talking to an idiot who doesn't know anything about what
pharmaceuticals are, how they work, what neurotransmitters are and who probably can't name a single region of the brain. I can describe it in detail,
and I'd still feel uneasy telling others what they're thinking or how it's working.
Ether is a fair bit worse than acetone, so you need to be careful using ultra cheap motors; as they tend to emit visible sparks continuously as they
spin. Not including a scrubber before the fan will make things worse (as I say in my reply to bob, the fumes will be going straight through the
motor). As a lot of those motors use the air going past to cool themselves, that's not great at all when something's potentially flammable.
Centrifugals at least have some degree of isolation between the motor and the blades. An oscillating fan, or wall fan, will often suck vapours back in
as they go past. If you can see 'grids' on the sides of the motor body, don't use it. Vacuum cleaner motors do this as well. Although, if you can put
up with listening to one of those for 6 hours, 3rd degree burns will likely be a godsend.
Like... oh my godz... fire!!!!!!
Ideally, everyone should have a CO2 & powder extinguisher, as they'll do just about all fires when combined. But particularly so if you're not
confident around flammables. You need the big kind as well. The small one's will barely cope with a B14 spill. I can promise you, my tiny acetone fire
is nothing like the reality of it all going up unexpectedly, you will want an extinguisher and the adrenaline will be rushing harder
than you've felt it for most of your life. And it will mean the difference between you loosing your home, people being scared for
life or dyling and just getting a fright. Best way to start understanding what you're dealing with, try setting fire to it on purpose, as I did above.
Then you'll appreciate, tangibly, how quickly it boils off and how it ignites.
[Edited on 11-8-2010 by peach]
|
|
|
| Pages:
1
2
3
4 |
|