| Pages:
1
..
20
21
22
23
24
..
30 |
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I will send you the requisite amount, remind me when we speak tomorrow.
|
|
|
Boron Trioxide
Harmless

Posts: 42
Registered: 18-6-2012
Member Is Offline
Mood: No Mood
|
|
Another quick question
What purity is Animal Feed Grade Material, and what might its impurities be?
Thanks
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
From what I can tell from a cursory search, from a purity standpoint, it's somewhere between technical and reagent grade. As for impurities, it
depends largely on the substance in particular and the industrial procedures used. Generally, the only impurities are those that either pose no or
little harm to animals, or lack the concentration to be dangerous.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Is gluconic acid very soluble in ethanol, even cold ethanol?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
http://pubs.acs.org/doi/abs/10.1021/ie50518a030
| Quote: |
It is extremely soluble in water, but only slightly soluble in [ethyl] alcohol, and it is insoluble in most other organic solvents.
|
|
|
|
gnitseretni
Hazard to Others
  
Posts: 280
Registered: 5-1-2007
Location: Medellin
Member Is Offline
Mood: No Mood
|
|
What do you do when the stirbars keep flying into the walls of the container? The smaller (1"-1.5") stirbars don't but they don't stir well enough.
The 2" stirbar I have would stir well enough if it didn't keep flying into the walls of the container. I'm trying to stir about 400ml of mixed acids.
I wanted to nitrate some methanol but it being so much lighter than the mixed acids it needs to be stirred pretty well otherwise it just floats on
top. I poured the mixed acids in a wider container and that seemed to help a bit, but still not enough.
Are oval stir bars better? What works best for mixed acids?
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Generally stirring is a non-issue for liquid solutions of low viscosity. If it's hitting the walls of the container in my experience the stirrer is
going too fast, or the container is convex. Usually I solve this by slowly raising the speed of the stirrer, hopefully this helps and aren't stupid
answers or things you've already tried.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 280
Registered: 5-1-2007
Location: Medellin
Member Is Offline
Mood: No Mood
|
|
My stirrer goes from 1 to 10 and the stir bar will hit the walls if I go past 7 regardless of how slow I raise the speed.
Anyways, I decided to do the nitration anyway. I used the 2" stir bar and a wider container. I had it stir as fast as it would go without the stir bar
flying against the walls. The vortex got smaller and smaller as I added more methanol, but it still must have stirred well enough because I got methyl
nitrate. Didn't think I would as it looked like the methanol stayed on top the whole time, but then again it was hard to tell as all liquids were
clear as water. Oh well, guess I worried too much 
|
|
|
Simbani
Hazard to Self
 
Posts: 50
Registered: 12-12-2012
Member Is Offline
Mood: No Mood
|
|
I have a quick question:
I wonder about the chemical stability of cyanoacrylates (glue). I really like to use this stuff to make my detonators gas-tight, but I don´t know if
there could be any
side reactions with HE´s of the following familys: nitramines, nitric esters, (peroxides), azides(lead and silver) and maybe stability towards
reduction (zinc, magnesium and so on).
And what are the products od decomposition?NOx?
[Edited on 24-12-2012 by Simbani]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
No idea on the last one, but I think it wouldn't be too resistant (considering it's basically Superglue).
My question: is potash pronounced pot-ash or poe-tash?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
Merriam-Webster says "POT-ash" and so do the Canucks, and I trust the Canucks.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
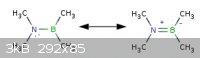
In "The Art of Writing Reasonable Organic Reaction Mechanisms" by Robert Grossman
the first problem asks which resonance structure is best. This one kind of confused me because I know boron to be an exception to the octet rule and
be "satisfied" with 3 bonds or 6 valence electrons. Ex: boron triflouride, borane, etc.
I said the structure on the left was the best using this knowledge because there is no charges making it 'preferred' but the author says the one on
the right is preferred because all of the atoms have a full 'octet'. Not sure who is actually right here but as you'll see even my chemical drawing
utility(Marvin Sketch) doesn't force a full 'octet' on boron.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Question: does anyone know generally how 'clean' "One Shot" drain opener/cleaner is, in terms of chemical purity? Does it usually have dyes in it, or
lots of suspended carbon?
Many thanks.
@Vargouille: I've always pronounced it as 'POT-ash', and IIRC several pseudo-scientific television shows have always documented it as 'POT-ash'.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by smaerd  | In "The Art of Writing Reasonable Organic Reaction Mechanisms" by Robert Grossman
the first problem asks which resonance structure is best. This one kind of confused me because I know boron to be an exception to the octet rule and
be "satisfied" with 3 bonds or 6 valence electrons. Ex: boron triflouride, borane, etc.
I said the structure on the left was the best using this knowledge because there is no charges making it 'preferred' but the author says the one on
the right is preferred because all of the atoms have a full 'octet'. Not sure who is actually right here but as you'll see even my chemical drawing
utility(Marvin Sketch) doesn't force a full 'octet' on boron. |
The title of the book, "writing reasonable...", is not there for nothing. If you have a B-N bond where boron has a vacant valence orbital and the
nitrogen a lone electron pair, it becomes unreasonable to expect no double-bonding interaction. It would be like writing the double bond of the
alkenes in the form of C+-C- rather than C=C. While such formality is advised when depicting ylides, nitro groups, sulfoxides,
phosphine oxides and the like (in order not to break the Lewis bond conventions), it is unreasonable to do elsewhere. Nevertheless, where writing the
more realistic type of interactions would make a mess, like in the case of non-localized B=N bonds, it is preferably avoided. For example, the B-N-B
or the N-B-N connectivity is better left depicted as it is.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
That makes a lot of sense thanks for clearing that up. For some reason I forgot that just because it can form 3 bonds does not imply that the empty
valence shell does not exist to interact in bonding or resonance. MO theory more than likely makes sense of that and I guess some common-sense I need
to adapt. Thanks again.
|
|
|
Glucose Oxidase
Harmless

Posts: 37
Registered: 31-12-2012
Member Is Offline
Mood: Researching Alchemy
|
|
Does C(NO3)4 exist?
please note that it is NO3 not NO2 so the bond between the radical and the carbon is via oxygen not nitrogen.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
No.
Also there is an article where they mention it... Also they made reactions with it, but I'm skeptic, because there is no prep mentioned anywhere.
Khisamutdinov, G. Kh.; Slovetskii, V. I.; L'vova, M. Sh.; Usyshkin, O. G.; Besyrozvannyi, M. A.; Fainzil'berg, A. A.
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1970 , p. 2397 - 2399
[Edited on 1-1-2013 by kristofvagyok]
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
I found an eBay seller in Poland who is offering 250mL of benzaldehyde for around $24 (including the shipping cost). Is this a good price for
benzaldehyde? Would it arouse suspicion if I purchased it?
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
Is it possible to go (easily) from formaldehyde to formic acid?
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
In theory, yes. Formaldehyde (or the solution of it, formalin) is an aldehyde, and consequently can be oxidized to formic acid (a carboxylic acid) by
refluxing with an oxidant, such as acidified potassium dichromate.
In practice, not quite. I've spoken with DJF90 previously and it seems that formic acid also has a tendency to be oxidize (it itself being a good
reducing agent) into carbon dioxide and water, so one would have to be very careful when preparing it via this route and I would imagine that
controlling it would be somewhat difficult and problematic.
[Edited on 4-1-2013 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
Thanks Hexavalent
[Edited on 5-1-2013 by learningChem]
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
What about using the canizzaro reaction? formaldeyde -> methanol + formate?
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
You are right. The Cannizzaro reaction would produce sodium formate and methanol. You could isolate the formate and distill it with sulfuric acid
(look for an actual procedure, this may not be ideal) If you want a lot of formic acid, you can buy it from Dudadiesel.com. It's not very expensive
($12 a liter or so).
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Quote: Originally posted by barley81  | | You are right. The Cannizzaro reaction would produce sodium formate and methanol. You could isolate the formate and distill it with sulfuric acid
(look for an actual procedure, this may not be ideal) If you want a lot of formic acid, you can buy it from Dudadiesel.com. It's not very expensive
($12 a liter or so). |
In theory it should work, the equilibrium being shifted to the right due to the formation of a weaker acid.
If you don't want to buy it online, it is sold in many hardware stores as a limescale remover. I bought a sample from a local store a few months ago,
and found it to be of 40% concentration, but remarkably pure (clear liquid, no colour at all, very few trace impurities).
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Question: why do filtrates tend to froth/foam during vacuum filtrations?
[Edited on 7-1-2013 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
| Pages:
1
..
20
21
22
23
24
..
30 |