| Pages:
1
..
5
6
7
8
9
..
40 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
SO3 crystals. Beautiful but treacherous!

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
These look great! Reminds me of my own experiments.
It does look a bit like white mold when the bottom of a flask is covered in crystallized SO3, with all these fine hairlike needles.
|
|
|
Megamarko94
Hazard to Self
 
Posts: 68
Registered: 31-12-2010
Member Is Offline
Mood: No Mood
|
|
copper nitrate cristals....
 
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
A few drops of iodine tincture in a flask with mineral oil and water.

|
|
|
Xenomorph
Harmless

Posts: 17
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
I was makeing some tetraammine copper (II) sulfate for furteher experiments with tetraammine copper salts... and was amazed that cystals grew so
beautiful and large!!!
 
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Wow, Xeno- Those are beautiful!
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i always loved that about iodine. different solvents different colors. and during sublimation the most intense purple vapor!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Xenomorph  | I was makeing some tetraammine copper (II) sulfate for furteher experiments with tetraammine copper salts... and was amazed that cystals grew so
beautiful and large!!!
|
Fantastic: a more detailed write up would be welcome here 
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Jor  |   
I was reducing a solution of (NH4)2PdCl6 (about 400mg Pd) in 25mL of 10-15% ammonia with hydrazine sulfate at 60-70C. A beautiful palladium mirror
formed, but I had no camera at hand. Shame, would have been a nice picture! |
I did something similar with a gold solution I formed a gold mirror in a flask, turning the bottom half of the flask (maybe ~25mL) into gold by
deposition of gold dust on the walls (a black powder when it was rubbed off). I didn't take a picture either. 
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
A gold mirror flask would be amazing! I have a silver mirrored Erlenmeyer
Maybe I can find a picture with Iodine vapor in it. My favorite!
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Oleic acid-capped cadmium selenide quantum dots suspended in toluene. Based on the UV-absorbance edge (558 nm), the Brus equation indicates that the
core-crystals are approximately 2.78 nm in diameter.
Cheers,
O3

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Nice picture! I"m sure that there are many interesting inorganic compounds that would look awesome under UV. I'll have to pull out mu old UV lamp out!
I see that you have some Pyrex Hach glassware. Do you have the volatile compounds distillation apparatus?
Hach Distillation Apparatus
I have two complete kits (minus hotplate) and would be willing to part with one, would trade for other glassware. 
Robert
[Edited on 15-6-2011 by Arthur Dent]
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
Xenomorph
Harmless

Posts: 17
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
Just left hot and really concentrated solution overnight at room temperature (~8 hours) in round-bottom flask where I made it (added excess of 25%
ammonia to hot saturated solution of CuSO4*5H2O and heated until all solid dissolved). There were no other small crystals, only these large ones in
picture...
[Edited on 15-6-2011 by Xenomorph]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks.
|
|
|
Xenomorph
Harmless

Posts: 17
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
Here is electrochemically crystalized silver. I did this few months ago by using electrodes of ~80%Ag/20%Cu alloy and AgNO3 electrolyte. I dont
remember exact voltage...
    
|
|
|
LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
Here is what I call the copper rainbow, even though the yellow is sort of yellow-green.
 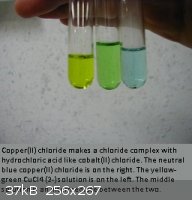 
[Edited on 16-6-2011 by LanthanumK]
hibernating...
|
|
|
sternman318
Hazard to Others
  
Posts: 121
Registered: 21-4-2011
Member Is Offline
Mood: No Mood
|
|
More physics than chemistry, I suppose, but here is my homemade Lichtenberg figure, or static discharge on powdered Zn on a ziploc bag
[Edited on 17-6-2011 by sternman318]

|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Finally found a picture of the home made iodine. So sparkly!

Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
1) copper coin in 65% nitric acid

2) fluorescein (sodium salt) solution under UV lamp

3)Rhodamine B solution under UV

|
|
|
spotlightman1234
Harmless

Posts: 17
Registered: 16-4-2011
Member Is Offline
Mood: No Mood
|
|
I have a decent picture of some tetraamine copper(II)nitrate, but I am not familiar on how to post an image... any help? sorry for the noob questions
 . .
|
|
|
LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
Click "Edit" on your post. Below the typing screen you will see photo uploading slots. Do not use the "Quick Reply" on the bottom; it does not have
all of the features.
hibernating...
|
|
|
spotlightman1234
Harmless

Posts: 17
Registered: 16-4-2011
Member Is Offline
Mood: No Mood
|
|
Thank you. For your kindness here is a picture of some tetraamine copper(II)nitrate I made a few months ago.

|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
I tried my hand at the macro-photography option of my cam to get this reflected image of
the beatiful structure of Copper Sulphate crystals that have grown in a supersaturated
solution. The original single crystal that was grown on a suspended copper wire in the
solution, was much bigger but sadly, these structures are so fragile that they crumble
very easily.

Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
plastics
Hazard to Others
  
Posts: 141
Registered: 6-11-2009
Member Is Offline
Mood: No Mood
|
|
Homemade glow stick!
TCPO, TCP and 9,10-diphenylanthracene (fluorescent dye) all synthesised by yours truly

|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very nice! I've done it with rhodamine B
Does diphenyl oxalate give the same light?
|
|
|
| Pages:
1
..
5
6
7
8
9
..
40 |