| Pages:
1
..
8
9
10
11
12
..
40 |
theflickkk
Harmless

Posts: 33
Registered: 29-3-2011
Member Is Offline
Mood: No Mood
|
|
Hmm Nick F, you could try changing the ISO to a higher value (may result in noise though)
Alternatively, the shutter speed can be changed by varying f-stop.
A slower shutter speed would allow more light to enter, making the picture brighter.
The sample you've got there looks really nice! Is the glow really so bright or is it just the camera o:
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|

A layer of 6-methylquinoline under water resulting from steam distillation.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice piece of art, UC.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Nice! I also love your new videos, UC
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Small crystals of rac. N,alpha-dimethyl-beta-phenethylammonium chloride.
(Ok, this should go to the ugly pictures thread, but there is none.  ) )
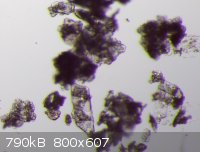 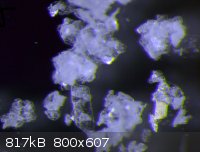
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Thanks to Nick F for the suggested type of UV lamp (found one for under $5 on e-bay). Comparison of uranyl nitrate, erbium acetate, and neodymium
carbonate under tungsten incandescent light and UV light.
 
Images of lead iodide. I dissolved 0.35 grams in 100ml of water, and boiled for about 10 minutes. The substance dissolved slowly, the solution
gradually becoming clear.
Cooling down, thin colourless platelets immediately formed on the liquid surface. After cooling down to RT, very many sparkling and deeply yellow
platelets had formed.
 
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
I enjoy the PbI2 golden rain, good job 
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|

Shattered glassy mass of 5-carboxypentyltriphenylphosphonium bromide (I think I named that correctly...) prepared from 6-bromohexanoic acid and
triphenylphosphine
The molten material is viscous and can be drawn into threads and so forth, like a proper silica glass, but at a vastly lower temperature.
The crystalline salt was prepared by precipitating the product from chloroform with ethyl acetate.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Wow! This is a realy nice picture! Are you doing a video to show the synthesis on Youtube?
[Edited on 24-12-2011 by plante1999]
I never asked for this.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by plante1999  | Wow! This is a realy nice picture! Are you doing a video to show the synthesis on Youtube?
[Edited on 24-12-2011 by plante1999] |
No.
1) It's very simple to prepare and can be summarized in the video where I use it for a wittig.
2) I was filming anyway, but I ran into a few snags along the way. And that makes for poor video.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Wow!!!
At least you can use some of the footage for the next video as a summary. looking forward to it
Synthetic capsaicin, sounds spicy
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I was attempting to "blue" some steel over the flame of my modified alcohol burner. I met limited success, as small areas of the piece of metal in
question turned to a corrosion resistant iridescent blue. My camera does not pick up the vivid colours of the blued metal, so I have no pictures to
display. But as I was washing the piece of metal clean, I found that the soot deposited on the piece of metal made the steel hydrophobic. It's nothing
special at all, as the layer of soot wears off very rapidly and does not stand up against abrasion even of the gentlest kind. But it sure does make a
nice picture I thought I might share with the community. Without further ado, here is my failure/success, several drops of water on the soot covered
bottom of a tin can, the cheapest way you can demonstrate the phenomenon of hydrophobicity.

"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by mr.crow  | Wow!!!
At least you can use some of the footage for the next video as a summary. looking forward to it
Synthetic capsaicin, sounds spicy |
I don't have enough footage to make it worthwhile, except maybe some shots of the same stuff I posted above. Synthesis pretty much consists of "dump
two reactants in flask, heat for 4 hours at 145C, dissolve in CHCl3, add ethyl acetate to precipitate, filter, wash, dry." It doesn't even have cool
apparatus.
Synthetic capsaicin is indeed my goal....I'll be running with dimsyl sodium in DMSO (heat sodium amide and dry DMSO together...I don't have any NaH,
but I can make NH3(l) and I have sodium on the way.)
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Your picture looks like a queer kind of sheet metal in a flask. Cool.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
A 7 mm crystal of p-dimethoxybenzene à la PainKilla that grew directly out of the reaction mixture. 

|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Drops of aniline condensing on the upper wall of an RBF while vacuum-distilling aniline. The attachment aniline.background needs to be renamed to a
.jpg and is a 1920x1200 image if you'd like to use it as a background. (This gets around the forum's size limits for pictures)
[Edited on 1-1-12 by UnintentionalChaos]
Attachment: aniline.background (730kB)
This file has been downloaded 1047 times

Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
Here is some pictures of a nickelocene, bis(cyclopentadienyl)nickel(II) synthesis I conducted at my home lab over Christmas break.

[Edited on 2-1-2012 by benzylchloride1]
  
[Edited on 2-1-2012 by benzylchloride1]
 
[Edited on 2-1-2012 by benzylchloride1]
  
[Edited on 2-1-2012 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Your selection of glass, chemicals and analytical equipment is unparallelled among amateurs, benzylchloride1. May I ask how you obtain your chemicals?
Seeing as you live in the US, most people run into a few problems on that front.
How did you synthesize the nickelocene? I have considered buying some dicyclopentadiene to prepare metallocenes myself. I'm guessing it's the
dicyclopentadiene you have in that solvent still?
Btw - I and many others would love if you'd write down something for the prepublication section. You obviously have many interesting, challenging,
novel (and legitimate) projects. I for one would love a topic summarizing your carpanone synthesis, as I find total synthesis to be probably the most
interesting subject in all of chemistry.
Edit: Oh, and those ampoules are simply lovely 
[Edited on 2-1-2012 by Lambda-Eyde]
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I obtain many chemicals off of Ebay, and I also know someone that I can buy chemicals through. I am thinking about putting a article together for the
prepublication section on the nickelocene synthesis since I really enjoy this prep. I used balloon grade helium as the inert gas in this synthesis.
The single piece solvent still was used to distill my THF from sodium benzophenone ketyl prior to use, The pictures do not do justice to the color of
the THF still pot. In this post there is a picture of the still that I used for cracking the dicyclopentadiene. I would recommend buying some
dicyclopentadiene since it is usually dirt cheap if you have a source for it and it is useful for so many things. I prepared nickel (II) chloride from
nickel (II) carbonate purchased on Ebay. From the nickel (II) chloride, I prepared hexaamine nickel(II) chloride using 30% aqueous ammonia, I bought
this from High Valley Chemical. The 10% stuff from Ace Hardware can be used, but it requires more acetone for crashing the product out. I would
recommend making this coordination complex to anyone interested in amateur chemistry. I produced a solution of sodium cyclopentadienide in THF by
adding cyclopentadiene to a stirred suspension of powdered sodium in THF. After the formation of the sodium cyclopentadienide, which is light purplish
pink in color, the hexaaminenickel(II) chloride is added and the mixture is slowly heated to reflux, resulting in the generation of a large amount of
ammonia gas and a gradual color change to the characteristic bright green color of nickelocene. The mixture was then filtered through pool filter aid
under helium, and the solvent removed under vacuum, leaving the crude product, which was then sublimed under dynamic high vacuum from a 80 C oil bath.
I would recommend perusing surplus stores of major universities on a regular basis, this seems to be the best way to build a lab very quickly.
Interesting that you brought up the carpanone synthesis since I have been working on this lately, I am very close to finishing it, I had to rethink
how to synthesize the sesamol. Take a look at the carpanone thread for an update. I am also thinking about writing an article on the preparation of
bis(1,2-diphenylphosphino)ethane and other chelating phosphines. I have been interested in these phosphines for making organometallics and
coordination complexes. There seems to be no report in the literature for preparing these compounds in solvents other then liquid ammonia. I ended up
using a patent for making sodium diphenylphosphide in anhydrous ethylenediamine for my purposes. After adding 1,2-dichloroethane, allowing the mixture
to stand overnight and a aqueous work up I got a 60% yield of the product. I tried the reaction twice on two different scales and the yields were
within six percent of each other. I am planning on preparing some of the corresponding phosphines from dichloromethane and 1,3-dichloropropane.
[Edited on 2-1-2012 by benzylchloride1]
[Edited on 2-1-2012 by benzylchloride1]

[Edited on 2-1-2012 by benzylchloride1]
[Edited on 2-1-2012 by benzylchloride1]
[Edited on 2-1-2012 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Chemiluminescence of 2,4,5-triphenylimidazole!
It's nothing special, but i like this compound!

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Running a Sodium Perchlorate cell (Lead Dioxide Anode) with a very high concentration of Perrchlorate and had a power cut. The temperature of the cell
fell from 50 to 30 degrees C and I obtained some nice shards of Sodium Perchlorate on the Anode. Most of them seem to come from the MMO just above the
Anode proper.

[Edited on 25-1-2012 by dann2]
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Wow, the NaClO4 is really beautiful! I wish I had some MMO electrodes, lol...
Rest In Pieces!
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Just some gray titanium tubing anodized/flamed with a propane torch. I found if you polish the tubing surface with a very fine sandpaper it produces a
far more vivid color pattern when the oxide layer forms. I think from memory, the first color to come up is a bronze-gold and then blue the more you
heat it. After that it's tricky to get the pinks and greens. If you are not careful and heat it too much, it reverts back to a drab gray with faint
colors barely seen. The best color is seen when viewed in indirect sunlight.

[Edited on 26-1-2012 by Morgan]
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Recently I did some copper electroplating / brass electroetching. After I was through I let the electrolyte evaporate naturally and this is what I
was left with.
- I know CuSO<sub>4</sub> has been done to death but this is all I have lately.

|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
A few more pretty pictures!
P
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
| Pages:
1
..
8
9
10
11
12
..
40 |