| Pages:
1
2 |
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Piranha solution should do the trick. Any organic substances that don't get washed away with solvents can be cleaned with concentrated sulfuric acid
and 30% H2O2
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Opylation  | | Piranha solution should do the trick. Any organic substances that don't get washed away with solvents can be cleaned with concentrated sulfuric acid
and 30% H2O2 |
Unfortunately, being in the EU, 30% H₂O₂ is not an option. Fortunately, I've found that dissolving sodium percarbonate directly into
conc. sulphuric acid was a more than acceptable substitute.
Thanks for that. Since I intend to try the reaction again with a fully dried thiourea, I won’t wash my bottle to the utmost cleanness, but I’ll do
that, eventually.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
You can make your own 30% H2O2 using the 3% from the store. It may not be as cheap as getting a jug of 30%, but you can boil the volume of liquid down
to 10% it's original volume for close to 30%. I've done it without issue. I usually try to keep it at a simmer or very light boil, not rolling boil.
It may degrade a bit during this process, but the result is plenty strong enough for piranha. Trust me
EDIT: also, make sure to do this in some very clean glassware. No metal containers as any bit of iron or other transition metals will catalyze
decomposition of H2O2
[Edited on 23-5-2021 by Opylation]
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Oh, I know! We can even get 12% here, so 1 L can be boiled down to 330 mL 30%+ hydrogen peroxide. If I'd do it, I'd use vacuum to lower the b.p. and
avoid most of the degradation.
TBH, sodium percarbonate doesn’t degrade with time, and is very handy to store. The only drawback is that dissolving it into any acid it releases
sodium carbonate with tends to neutralise the acid, but with concentrated sulphuric acid it’s a minor issue.
Besides, one of my next experiments will be an hydroboration of styrene to get phenethyl alcohol (something I've been wanting to synthesise for a long
time), and the final quenching step, which involves oxidation of the intermediate by hydrogen peroxide under basic conditions, can conveniently (at
least I think) be created by adding portions of sodium percarbonate.
[Edited on 23-5-2021 by Keras]
|
|
|
garphield
Hazard to Self
 
Posts: 58
Registered: 9-12-2019
Member Is Offline
|
|
Cyanamide will react with hydrogen sulfide to generate thiourea (which will thermally decompose to generate CS2 among other things, source is attached
pdf). What would the products of the reaction between molten sulfur and calcium cyanamide be? Molten sulfur isn't great but it is both easier to
produce and less unpleasant than either H2S or SxCl2.
Attachment: thiourea_thermal_decomposition.pdf (169kB)
This file has been downloaded 279 times
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
Quote: Originally posted by Keras  |
Oh, I know! We can even get 12% here, so 1 L can be boiled down to 330 mL 30%+ hydrogen peroxide. If I'd do it, I'd use vacuum to lower the b.p. and
avoid most of the degradation.
|
No need to boil I think, what you need to do is freeze. there are videos on the subject on YT.
"A mind is a terrible thing to lose"-Meisner
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 151
Registered: 12-7-2018
Member Is Offline
|
|
Or without loses.You can dry it. one big baker or vakuuexicator filled with h2so4 or other high hygroscopic material and an smaller baker with
h202.Stored in an dark place. Some guy had an youtube video too he needs little bit over 70days to reach 75%.
https://www.youtube.com/watch?v=0vcbZQHcPWU&t=151s
only in german
[Edited on 1-6-2021 by Alkoholvergiftung]
[Edited on 1-6-2021 by Alkoholvergiftung]
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Lol. Genau, das stimmt. :p
But you have to be very patient, or plan it quite in advance!
(Besides, with sulphuric acid now in short supply, that method is going to lose its appeal, I suppose).
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Quote: Originally posted by garphield  | | Cyanamide will react with hydrogen sulfide to generate thiourea (which will thermally decompose to generate CS2 among other things, source is attached
pdf). What would the products of the reaction between molten sulfur and calcium cyanamide be? Molten sulfur isn't great but it is both easier to
produce and less unpleasant than either H2S or SxCl2. |
That paper is very interesting. The only issue there is how do you get the CS2 to not react with ammonia? Maybe add an alkoxide into the mix to form a
xanthate? Or maybe alkylate the Thiourea so that the reaction forms a dithiocarbamic acid so after isolation you can decompose it a lower temp and
hopefully distill CS2 out?
Or maybe it’s even easier than that. Feed the gaseous products into hydrochloric acid and then use a sep funnel to obtain carbon disulfide
[Edited on 4-6-2021 by Opylation]
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Opylation  |
That paper is very interesting. The only issue there is how do you get the CS2 to not react with ammonia?
Or maybe it’s even easier than that. Feed the gaseous products into hydrochloric acid and then use a sep funnel to obtain carbon disulphide
|
Would CS₂ and NH₃ react?
As I explained, I heated thiourea, and passed the gaseous products produced first into a Drechsel bottle full of mineral oil, then another full of
hydrochloric acid. Got what I think is NH₄Cl, though I am not sure, because the crystals I collected after evaporating the HCl are white, but more
“fluffy” than I imagined. There was no trace of CS₂ whatsoever, albeit the gases were led to the Drechsel bottles though a cold trap.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Ammonia and Carbon Disulfide react to form ammonium thiocyanate and hydrogen sulfide. However, what I didn't notice until I was getting this screen
grab is that ammonium thiocyanate is in equilibrium with thiourea, which means that any ammonium thiocyanate can just be converted back to the
starting material. Even further down, it states that my concerns aren't even warranted as ammonium thiocyanate decomposes to CS2, NH3, and H2S
Also, would the carbon disulfide no dissolve in the mineral oil? They're both non-polar molecules. I would omit the mineral oil and feed it straight
into HCl acid to remove the ammonia
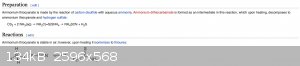
[Edited on 5-6-2021 by Opylation]
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Opylation  |
Also, would the carbon disulfide no dissolve in the mineral oil? They're both non-polar molecules. I would omit the mineral oil and feed it straight
into HCl acid to remove the ammonia |
Ha. Gosh. I didn't even think about that. I was worried CS₂ could somehow react with HCl or water. As you say, I should probably first scrub the
ammonia using HCl, then led the exhaust into something that dissolves CS₂. However, I fail to understand why the carbon disulphide did not condense
into the receiving flask, despite it passing through a Liebig condenser and that flask, both being cooled by ice cold water.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
I’m not quite sure. I haven’t performed this reaction but have logged it for when I have time to try it. It might require heating in a stream of
HCl to lock up the ammonia. The decomposition temperature for ammonium thiocyanate is 200C which is well below the decomposition temperature of
ammonium chloride. A short path condenser may alternatively work or even a takeoff adapter from the reaction flask to a gas scrubber filled with HCl
acid. It’ll probably need some trial and error unless you can find a good paper describing the exact result you’re looking for.
Also carbon disulfide does hydrolyze but not readily and should be able to handle water for brief periods
[Edited on 7-6-2021 by Opylation]
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Unfortunately, I had no other details about the reaction but the fact that, around 220 °C thiourea under argon atmosphere decomposes into ammonia and
carbon disulphide. A reaction certainly takes place, and, as indicated by the pictures I took, it also generates elemental sulphur (albeit in very
slight proportion) which deposits on the cool walls of the glassware, threatening to block it, and is quite a pain to remove afterwards (needs hot
toluene washing).
I’m wondering if that sulphur deposit was not caused by traces of water in the thiourea. If you try that reaction, I'd advise you starting from
dried thiourea, i.e. by having it baked in an oven for an hour or so.
|
|
|
garphield
Hazard to Self
 
Posts: 58
Registered: 9-12-2019
Member Is Offline
|
|
The products of the reaction between calcium cyanamide and sulfur might be higher in sulfur and therefore yield more carbon disulfide. Additionally,
there would be no hydrogen so you wouldn't have to worry about ammonia, and there would be no need to work with H2S gas. Could someone with more
knowledge about chemistry than me predict what the products of that reaction would be?
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 250
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
I made CS2 a while ago in a way I thought was pretty convenient.
I used the fact that charcoal is a pretty decent insulator to my advantage. Filled a 1 liter round bottom flask with a mix of activated charcoal and
sulfur. Activated charcoal because it has a convenient and consistent particle size, is generally quite clean and not dusty, and I would imagine a
little less hydrogen overall than most charcoal to minimize unwanted H2S production.
Then I simply lowered an electric heating element(100-200 W ish) into the flask and positioned it to be as far from the flask walls as
possible(center) and surrounded by the charcoal.
The charcoal insulates very well and protects the flask from getting excessively hot, and allows maintaining the high temperature in the middle quite
easily and with relatively low power.
Ideally the power or insulation would be regulated to condense but not freeze the sulfur on the inner walls, allowing it to pool at the bottom of the
flask and circulate through the hot zone without getting into the condenser meant for the CS2.
It worked for small amounts of CS2, but there's room for improvements. The heater element is very much a consumable like this. I don't think any metal
can survive being red hot in a sulfur atmosphere...
Dipping the element in glass frit to form a viscous glass layer on it helped and could probably be refined further. A halogen bulb as a heat source
might work too.
I was hoping the charcoal would be conductive enough to simply use electricity to heat the charcoal resistively but based on my attempts this would
require a voltage on the order of 500-1000 V to work reliably, which makes it less convenient and more dangerous...
Another interesting way that works great in theory is to use carbon fibre or graphite rods as the heater element. I tried with carbon fibre but I had
problems figuring out a good way for a reliable electrical connection; it kept burning off near the connection or coming undone. An upside with this
method is it should keep H2S production very low since afaik there's little to no hydrogen present in graphite.
[Edited on 17-6-2021 by Junk_Enginerd]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | I don't think any metal can survive being red hot in a sulfur atmosphere... |
What if it's already yellow? 
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Quote: Originally posted by macckone  | Bryce, W. A.; Hinshelwood, Cyril (1949). 707. The reaction between paraffin hydrocarbons and sulphur vapour. Journal of the Chemical Society
(Resumed), (), 3379–. doi:10.1039/JR9490003379
This produces a lot of crud but the three main products are hydrogen sulfide, unsaturated hydrocarbons and carbon disulfide.
Bubbling acetylene vapor through molten sulfur seems like a viable method. Acetylene can be easily produce from calcium carbide. This will reduce
the amount of hydrogen sulfide that is produced. 77% of the product is carbon disulfide at 325C. I would expect you could add a catalyst and increase
the reaction rate. The paper below tried iron and iodine with little effect. Silicate might help. This is one of those research topics.
http://www.sciencemadness.org/talk/files.php?pid=153376&...
Regardless of method flammability is an issue. I would recommend carbon dioxide as an inert gas as it is easy to produce and far cheaper for the home
chemist than argon.
|
here I found a video of the CS2 synthesis from acetylene + sulfur
https://www.youtube.com/watch?v=71njL6POaek
H2S produced as a byproduct, be careful !!!
Seems they displaced the air from the apparatus by the acetylene itself before starting heating - also a clever idea.
|
|
|
| Pages:
1
2 |