zeppelin69
Harmless

Posts: 46
Registered: 20-2-2007
Member Is Offline
Mood: No Mood
|
|
Desymmetrization through chiral catalysts.
This summer I was fortunate enough to land an undergrad research position in an organic lab at the university I attend. My job up until now has been
building up starting materials for a grad student who has been struggling with his project for a while, but recently my professor decided that my time
would be better spent working on my own project. I gave him a few of my own ideas for what I could do, and he liked one of them so much that he told
me to get started right away. I was pretty excited at first, but I am realizing now that I'm in over my head.
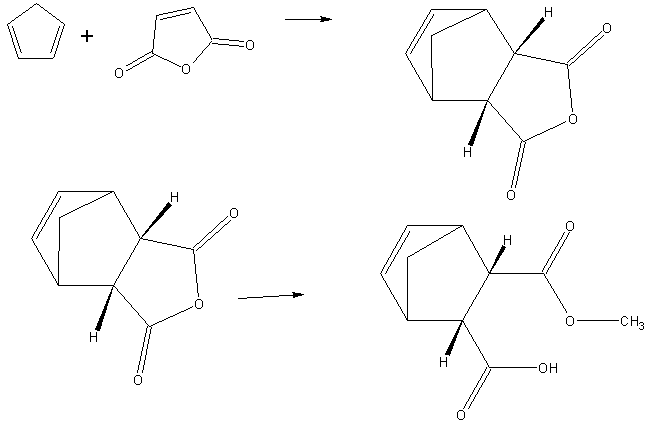
The basic idea is to make the Diels-Alder product of cyclopentadiene with maleic anhydride, then use a chiral catalyst to split open the maleic
anhydride ring asymmetrically. The broken ends of the ring should have selectivity for which groups they get (one being an ester and the other
remaining as the acid), so this way I would have control over the stereochemistry in the proceeding steps. Then I can modify each of these chains
separately, eventually leading to intra molecular metathesis reactions and so on.
The problem is that I rushed into this project without doing enough research into these chiral catalyst. Does anyone on this board have any
experiences with them? More specifically, I am looking at thiourea based catalyst like these.
http://en.wikipedia.org/wiki/Thiourea_organocatalysis
Would it be feasible to attempt to make my own catalyst? Any advice or insight would be greatly appreciated.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
You may be in over your head. I don't know about you, but I do my most transcendental and amazing work when I'm on the verge of a nervous breakdown.
Don't ever be afraid to push yourself...hard.
Anyhow, I have no experience with these particular catalysts. What I would do is first of all, a lot of brainstorming. Go back to the fundamentals:
how do catalysts work? By a) raising the free energy of the reactants or b) lowering the energy of the relevant transition state, leading to
favorable yields of one product over another (via Curtin-Hammett principle) or increased reaction rate. In any case, I would spend a lot of time
thinking about how your transition state will look (the one that connects your diels-alder product to your cleaved ring) and how you can stabilize
that transition state with your thiourea catalysts.
I hope that helps a little, or at least gave you a warm-fuzzy feeling. Good luck!
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The desymmetrization of symmetric succinic anhydrides should already be described in the literature, so the first thing you should do, is to do a
thorough literature search and review.
I doubt chiral catalysts that interact with the substrate via H-bonds would do much in this case, but you can always try (but don't use solvents that
compete as H-bond acceptors or donors!). What you need are catalysts that interact with the carbonyls and modify their electrophilicity. Chiral Lewis
acids should do this best. There are many that are commercially available and others that can be easily made (like BINOL + AlCl3 or Ti(OiPr)4, etc.).
Consider also that the solvent should not be competing with the catalyst (thus destabilizing the transition state) or else you will lose on the
enantiomeric excess (if there is any at all), so you should not just use methanol as the solvent for the asymmetric methanolysis. Using an inert
solvent like CH2Cl2 and only a slight excess of MeOH would probably give better results. In short, for a good e.e. you will need good reaction
conditions optimizations, so you should not discard catalysts that initially give poor results without evaluating what causes good or poor results.
Like for everything, you need to actually understand what is going on.
Methanolysis of anhydrides can be catalysed by tertiary amines and DMAP-type of bases as well, so you might also consider testing chiral bases like
the ones of the cinchonidine-type. In any case, only those catalysts which form a diastereoisomeric transition state can induce an asymmetry in the
reaction outcome, so you can predict which chiral reagents can not act as desymmetrization catalyst by considering the structure of the transition
state for the catalysed methanolysis of your substrate, for which you need to know the exact mechanism of catalysis (which you can mostly find in the
literature for the related achiral catalysts - luckily there are only about three mechanisms).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
zeppelin69
Harmless

Posts: 46
Registered: 20-2-2007
Member Is Offline
Mood: No Mood
|
|
Thanks for the insight and advice. I was able to find a publication that was extremely relevant, and it's been helpful, but I am still lost on how I
should go about obtaining the catalyst. It seems too me that they aren't being sold anywhere, but that's just as well because I doubt my research
group could afford it and I like to make as many of my own reagents as I can anyway.
Just looking at catalyst 1 in the pdf, I wonder if I were to attempt to synthesize this myself, could I substitute the trifluoromethyl groups for
something like nitro? I assume that the CF3's are being used for their electron withdrawing properties, and considering my lab's financial situation,
it would be great if I could use a cheaper EWG.
Attachment: CC1.pdf (780kB)
This file has been downloaded 668 times
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Quote: Originally posted by zeppelin69  |
Just looking at catalyst 1 in the pdf, I wonder if I were to attempt to synthesize this myself, could I substitute the trifluoromethyl groups for
something like nitro? I assume that the CF3's are being used for their electron withdrawing properties, and considering my lab's financial situation,
it would be great if I could use a cheaper EWG.
|
It's true that both nitro and trifluoromethyl are electron-withdrawing groups, but the mechanism by which they do that is very different.
-NO<sub>2</sub> withdraws electron density primarily through resonance while -CF<sub>3</sub> withdraws electron density
primarily through inductive effects.
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
zeppelin69
Harmless

Posts: 46
Registered: 20-2-2007
Member Is Offline
Mood: No Mood
|
|
I understand how they are different and why, but do the differences between the two matter for this purpose? The point of using the trifluoromethyl
groups here is to pull electrons to one side of the molecule and create some polarity, is it not? If that is the case, then I shouldn't I be able to
use any strong EWG? I could imagine there being a problem if nitro groups were significantly more bulky than CF3, creating some hinderence and not
allowing the catalyst to "fit up" to the anhydride properly, but I imagine that they are relatively similar in size. Is there something else I am not
seeing that wouldn't allow this to work? I should probably mention that I have not yet completed my second Ochem lecture course, so I still have a
great deal of basics left to learn...
EWGs aside, for synthesizing the rest of the catalyst L-valine would probably be the most economical starting point, but I am at a loss for where to
go from there. At some point, I would have to form a dimethylamine group in place of the carboxylate on the amino acid. This would be simple if I were
using an acyl chloride. I would just form the amide and reduce to the amine, but as far as I know, there aren't any protecting groups that would allow
me to halogenate an amino acid to some sort of amino acyl chloride for this to work. What I find the most baffiling at this point though, is how in
the world do I attach the amino acid (or its derivitive) to an aromatic ring with thiourea inbetween  . I can see how adding the thiourea group to my amino acid alone wouldn't be too difficult, but if I were to do that
how can I attach the NH2 to the aromatic ring afterwards? . I can see how adding the thiourea group to my amino acid alone wouldn't be too difficult, but if I were to do that
how can I attach the NH2 to the aromatic ring afterwards?
Thanks again for the input so far DDTea and Nicodem .
[Edited on 7-11-2010 by zeppelin69]
|
|
|
zeppelin69
Harmless

Posts: 46
Registered: 20-2-2007
Member Is Offline
Mood: No Mood
|
|
I could really use a little help with the synthesis of this catalyst. I'm not looking to be spoonfed, I'm just not familiar with the reactions used to
make something like this. I'd really appreciate it if someone could point me in the right direction. Thanks again.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Truthfully, zeppelin, I'm not familiar with this either! As my adviser, a theoretical chemist, once said: "If two things look different, they
probably are." In regard to -CF<sub>3</sub> vs. -NO<sub>2</sub>, I genuinely do not know; I would have to thoroughly peruse
the paper and do the same research you would. However, the best pointer that I can give you: don't take anything for granted. If something looks
funny, investigate it. Don't make assumptions and if you do, at least know what assumptions you are making (after all, assumptions define the limits
of every model).
As far as synthesizing the catalyst goes: could you use some derivative of thiocarbamic acid, with L-valine attached to the nitrogen, in your
synthesis? Again, I'm taking a shot in the dark.
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|