Reduction of esters using calcium in a dissolving metal reduction
The following appears to be an alternative to the Bouveault-Blanc Reduction of esters to alcohols. Despite chasing the references given in the following article, I can't find much information on
this reduction. Anhydrous ammonia is probably not necessary for this reaction, since other dissolving metal reductions utilizing calcium take place in
ethylenediamine. (Those reductions may require vigorous overhead stirring and an abrasive compound like sand.)
I've attached two articles, one about Birch type reduction using calcium in methylamine/ethylenediamine and the other is the reference for this
reaction. I have some more articles that I can post if there is interest.
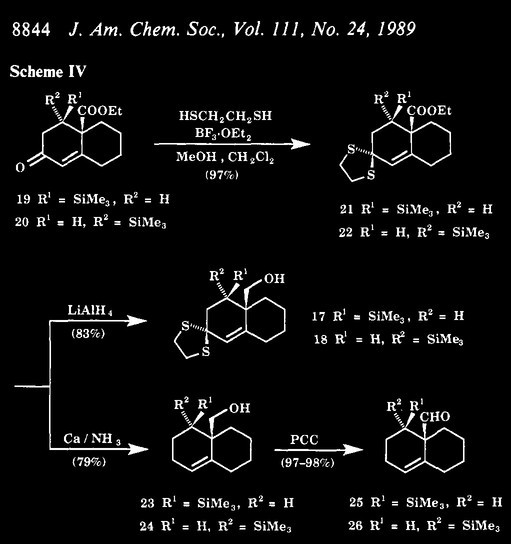
| Quote: |
Calcium metal (2.79 g, 69.8 mmol, 26 equiv) was dissolved in
liquid ammonia (45 mL) at -78 "C under an atmosphere of argon in a three-necked flask equipped with a Dewar condenser containing dry ice and acetone.
To the dark blue solution was added a solution of a mixture of ethylene thioacetals 21 and 22 (987 mg, 2.66 mmol, 1.0 equiv) in THF (20 mL). The
cooling bath was removed and the deep blue solution was refluxed for 5.5 h. Solid NH4Cl and diethyl ether (20 mL) were carefully introduced into the
reaction flask and ammonia was allowed to evaporate. Saturated aqueous NH4CI was added to the residue, and the aqueuos layer was extracted with two
portions of ether. The combined ether
solutions were washed with brine, dried over anhydrous MgS04, filtered, and concentrated to give an orange-pink oil. The oil was purified by use of
Chromatotron (4-mm plate, gradient solvent system of 5-10% EtOAc
in hexanes as eluant) to afford 23 in 32% yield (202 mg, 0.846 mmol) as a colorless oil and 24 in 47% yield (284 mg, 1.19 mmol) as a white solid.
|
Attachment: A new reducing system calcium metal in amines. Effect of hexamethylphosphoramide on calcium reductions.pdf (332kB)
This file has been downloaded 537 times
Attachment: Silicon-Directed Decarbonylation of Trans Trimethylsilyl Formyl Octalins.pdf (1.1MB)
This file has been downloaded 469 times
[Edited on 6-11-2010 by Bolt]
|