Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Tryptophan decarboxylation improved
I was looking for advice on the tryptophan decarboxylation and how to improve it. I have used d-pulegone in pure turpentine and had good results. Only
problem is that not everything dissolved and the solution did not become clear. I have read a few more papers and they report a 71% yeld with pulegone
and white spirits vs 65% using terpentine. I am ready to try this reaction and have pure carvone. It makes sense that this would work better because
the ketone is less sterically hindered by the alkene functional group than pulegone, since it is subsituted on the opposite side( look at structures
and do mechanism for schiff base formation). Has anyone had any insight or improvements. Also what is the best easily available source for white
spirits, since the term is so ubiquitous. They report a 95% yield using carvone in 1-hexanol, but hexanol is so expensive. I'd appreciate any advice.
As far as isolating tryptamine, i have been successful in isolating and purifying it via heptane ( didnt run column due to cost of silica and
chloroform/DCM). I also was able to make the HCL salt by dissolving impure product in methanol, adding conc. HCL, cooling to 0 C and slowly adding
ether with agitation. Any advice or experimental results wpuld be appreciate. I just want a cheap, otc method to produce a large quantity. I have
graduate school level chemistry experience.
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Heres a mechanistic sort of chair conformation geometry picture.
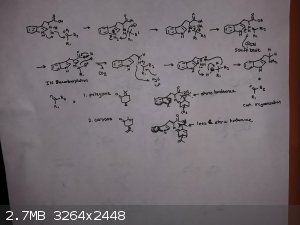
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Tryptophan and acetophenone should equal tryptamine.
Acetophenone should be the best decarboxylating agent with highest yeilds
[Edited on 18-5-2019 by draculic acid69]
|
|
|
monolithic
Hazard to Others
  
Posts: 435
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
I take it you've seen these two papers? 10.1016/j.chroma.2008.09.036 10.1016/j.jpba.2006.02.007
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Yes, i was basing my experiment of those papers! Ill look into acetophenone, but i believe it was too expensive so i chose a differwnt route
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
There have been a number of threads about tryptamine from tryptophan on this forum.
Comparisons of yield, discussion of theory, and some suggestions about purification.
The search engine here isn't the best, but if it doesn't give you good results search with Google for the subject but add Sciencemadness to the search
terms.
I believe this is a common procedure here.
The long-absent Aga used to recommend it to new members.
That's where I picked it up.
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Thanks. Yeah ive been able to get it to work no problem. Just wanted feedback from anyone with insight.
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
This reaction is very unreliable. Some reactions yield garbage and dont become clear upon conpletion, and other times the solution will become cleat
after 2 hours. This is with evacuated and nitrogen filled atmospheres.
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Got the last reaction to work. Used an old mother solution with a mix of 2 types of terpentine spirits and used excess pulegone. Heated it much more
than before ( need graham condenser for better condensation) and stopped after 6 hours and almost completely transparent.

|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Using turpentine and pepper mint oil is probably the source of the unreliability unless the tryptophans impurities are also a contributing factor.i
imagine these 3ingredients vary from batch to batch.
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Found Klean Strip turpentine works best. Its 70% (-)-alpha-pinene. Also the used turpentine from the previous solution is reused ( contains residual
pulegone) and this works very well. This is the 3rd type of turpentine used and it works amazing. 25g scale decarboxylation finished in under 3 hours.
Used 25g L-tryptophan(99.9% USP grade), 3.5mL pulegone ( 99.95+% purity), and 100mL previously used turpentine solvent(washed with AcOH, Na2CO3, NaCl,
H20, dried over MgSO4) + 150mL Klean Strip Turpentine.
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Beginning of reaction and end. Pictures of workup.
  
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
what kind of solubility in heptane are you seeing for tryptamine? Also acetophenone isn't terribly hard to make but it depends on what quantity you're
looking for.
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
I dont use heptane at all for tryptamine. I recrystallized with hexane and some ethyl acetate, but it is poorly soluble and only somewhat soluble in
boiling hexane, which is used to purify the tryptamine. Bring a small aliquat of hexane to boiling im tryptamine, swirl or agitate a bit and quickly(
but gently) decant into another flask and as soon as the hexane touches the flask and pools, it becomes milky white. Repeat this with several
aliquats(7×10mL) of boiling hexane roughly per gram of crude material. Set pooled hexane aside in freezer and after 3 hours, white crystals nucleate
and grow on the walls of the flask.

|
|
|
Kevlar
Harmless

Posts: 16
Registered: 5-2-2024
Location: UK
Member Is Offline
|
|
Is the use of DCM in place of Chloroform a good solvent choice?
And would I be correct in thinking any Typtophan than results with nasty tar in RXM, is due to the starter material being cut with binders? I have cas
numbered Tryptophan to test that is reagent grade so, guess I could give the answer for that after the next run.
If this is the case that the 500g of "suppliment grade" crap I have is full of other crap. Does anyone have any ideas of common binders/fillers and
how one might purify that to more pure Tryptophan.
I'm running these under Argon gas using Students Tech-

|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
Good results are obtained by doing the decarboxylation in pine turpentine with 5% DMSO added as co-solvent, and carvone as the catalyst. People have
reported succes using caraway or spearmint essential oils as the source of respectively D- or L-carvone. I've used pure D-carvone isolated from dill
seed oil. The addition of a small amount of DMSO seems to result in a much more reproducible process. Strong stirring is also important. The reaction
is over when no more tryptophan is in suspension.
There is no need to mess around with chloroform or DCM. Take advantage of the solubility of benzoic acid in organic solvents to directly precipitate
crude tryptamine benzoate from the clear post-reaction mixture (after cooling down to room temperature). For every gram of tryptophan you used, add a
gram of benzoic acid dissolved in a minimal amount of acetone to the mixture. The crude tryptamine benzoate can be recrystallised with acetone/hexane
to obtain a pristine pure product.
(eg. dissolve 1.5 g crude tryptamine benzoate in 100 ml boiling 20:80 acetone:hexane, let cool to room temp, chill in freezer overnight). Tryptamine
benzoate is stable and not hygroscopic.
The bulk supplement grade tryptophan powder is usually pure as stated in my experience, it seems you are running into problems in the work-up process,
which can be frustrating using the convential approaches. That is because tryptamine base has anomalous properties such as high solubility in water,
and attempts at forming salts using mineral acids lead to excessive decomposition as tar and colored impurities are formed. It is best to avoid
aqueous solutions of any acids altogether, like the standard A/B extraction with vinegar and such.
solubility of benzoic acid in acetone at room temperature : 28%, in toluene 9%, in xylene 11%, it is also sparingly soluble in naphta and mineral
spirits.
EDIt: Also, use an oil bath for better heat transfer. Peanut oil works fine for the required temperature 160-170°C.
[Edited on 12-3-2024 by dicyanin]
sic transit gloria mundi
|
|
|
walruslover69
Hazard to Others
  
Posts: 216
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
Read my thread on an improved synthesis/purification
as dicyanin stated, forming the benzoic acid salt is the best way to go. Based on how cheap high purity tryptophan and the solvents are. I don't see
there being much benefit in improving the yield,
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
|
|
|