CouchHatter
Hazard to Others
  
Posts: 146
Registered: 28-10-2017
Location: yes
Member Is Offline
|
|
Hydrochloric acid causing HDPE to bubble
I have a gallon of Crown HCl from my local hardware store. It has been tinted yellow-green since I bought it several years ago. I used about half of
it and it has been stored for about 2 years. I read the discoloration is from heavy metal contaminants but I haven't verified this. It was stored for
a summer in my shed but now it is stored indoors.
Today upon taking inventory I notice that the container has formed bubbles on the outside of the HDPE jug, something that definitely wasn't present
when it was moved inside. The cap has probably been off for <5 minutes its entire life. It fumes whenever the lid is removed but it has always done
that.
Is this normal? I will obviously replace the jug as the bubbles are soft to the touch and probably incredibly thin. But on all the compatibility
charts with 50% HCl, it shows HDPE as having good resistance. Maybe I've exceeded the limits of normal use. It's stored in a cabinet with other acids
- boric, sulfuric, acetic. None of the other containers show any sign of decomposition.

|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Guesswork ... HCl is a small molecule and it is infamous for escaping and rusting iron/steel etc.
You could check if it is HCl gas, migrated through the wall,
by putting a little ammonia soln. in a syringe then sucking the gas from the bubbles into the syringe via a needle,
white smoke in the syringe would be a positive indication.
(HCl trapped by a surface film, forming bubbles due to internal pressure being higher than atmospheric,
I can see that the surface film is not the label,
but it could be some kind of surface treatment or other manufacturing artefact)
P.S. if the bubbles are HCl due to internal pressure,
the implication is that you have a very good cap 
on a not so good bottle 
[Edited on 22-5-2019 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
I've seen that a couple of times on old hardware store HCL bottles usually accompanied by some salt on the bottle.
Not sure these standard bottles are HDPE.
On the other hand last week I used what I thought would be crap HCL for chlorine production. I had 4 bottles from a UK supplier that had been sitting
in a box for 2-3 years.
The bottles looked bad on the outside: part discolored, part yellow, part opaque etc but the acid inside was still clear and fuming.
I discovered that packaging chips really dont like HCL vapor 
|
|
|
CouchHatter
Hazard to Others
  
Posts: 146
Registered: 28-10-2017
Location: yes
Member Is Offline
|
|
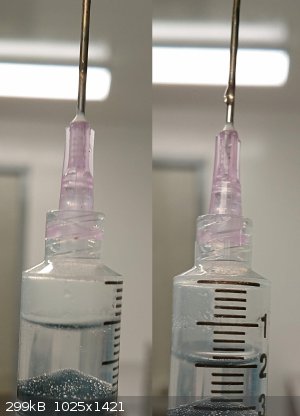
Sulaiman, the bubbles are tiny for my syringe tip but I did manage to carry out your suggestion. Thank you! Upon puncturing it let off a small wisp of
white gas, and I got most of it into the syringe. After emptying the syringe to verify it wasn't just clouded plastic, I could see a stream of white
gas exit the tip.
Herr Haber, do you mean virgin HDPE? Impurities could leach from the material which would explain why some hardware store HCL is clear and some are
yellow (not old enough yet). I took what I read long ago to mean that the HCL itself was contaminated from the start, but that makes more sense.
The HCL is now in an identical bleach jug.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Funny you should mention this as I just noticed a similar issue with HCl and a plastic bottle which is possibly HDPE.
I opened my box of halides and noticed that a the aluminium crimp on the top of a vial of tantalum chloride was corroded. Prime suspect was the
anhydrous AlCl3 in a (HDPE??) bottle in the same box, which on sniffing, did externally smell slightly of HCl. Then I noticed weird bubbles quite
similar to that in the bottle, and also on some other nearby (probably HDPE) bottles.
|
|
|
monolithic
Hazard to Others
  
Posts: 435
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DavidJR  | Funny you should mention this as I just noticed a similar issue with HCl and a plastic bottle which is possibly HDPE.
I opened my box of halides and noticed that a the aluminium crimp on the top of a vial of tantalum chloride was corroded. Prime suspect was the
anhydrous AlCl3 in a (HDPE??) bottle in the same box, which on sniffing, did externally smell slightly of HCl. Then I noticed weird bubbles quite
similar to that in the bottle, and also on some other nearby (probably HDPE) bottles. |
I also recently noticed HCl leaking through its shitty plastic bottle. Well, I noticed when my hands started to sting and I realized the sticker over
the bottle looked wet. Guess I need to transfer it to a glass bottle...
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by CouchHatter  | I have a gallon of Crown HCl from my local hardware store. It has been tinted yellow-green since I bought it several years ago. I used about half of
it and it has been stored for about 2 years. I read the discoloration is from heavy metal contaminants but I haven't verified this. It was stored for
a summer in my shed but now it is stored indoors.
Today upon taking inventory I notice that the container has formed bubbles on the outside of the HDPE jug, something that definitely wasn't present
when it was moved inside. The cap has probably been off for <5 minutes its entire life. It fumes whenever the lid is removed but it has always done
that.
|
I have some HCl in its original plastic bottle from BDH (a large chemical supplier). As BDH was renamed about ten years ago the bottle may be about
ten years old. The bottle has developed blisters. The bottle looks like polypropylene.
I am surprised that BDH would supply a chemical in a bottle that degrades in tens year. I would expect that would have been researched by BDH. OK I
don't know the storage conditions for most of those years and they may have advised glass for long terms storage.
I suspect the blisters are due to impurities in the plastic that under the influence of the HCl defusing to it produced the blisters or perhaps a
void in the plastic that with temperature cycling expands to a blister.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Per this source at https://www.servicethread.com/blog/the-uv-resistance-of-poly...
"The light causes the bonds holding the polymer together to break which weakens the plastic. This makes polypropylene unsuitable for uses that require
long term exposure to sunlight"
So, the bubbles are actually leaking HCl as the polymer degrades especially with natural sunlight (or via artificial source producing UV).
Interestingly, at one point when the bottles were outside, there could have been UV exposure (direct or via diffused sunlight) that weakened the
polymer. In time, this structural fault subsequently became evident as surface bubbles (relatedly, people exposed to sunlight can produce DNA damage
to their skin and subsequent issues).
[Edited on 8-7-2019 by AJKOER]
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Your link is dead or is in my part of the world
It just occurred to me I will have to check how even the bubble pattern is. That may give insight into if light is a significant effect. The bottle
bottom was probably not exposed as much as the sides.
[Edited on 8-7-2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks wg48temp9!
It is fixed now, I accidentally added ':' to the end of the link.
------------------------------------------------------------------------------
Good idea to check spots not as likely light exposed (including behind any labels).
[Edited on 8-7-2019 by AJKOER]
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by AJKOER  | Thanks wg48temp9!
It is fixed now, I accidentally added ':' to the end of the link.
------------------------------------------------------------------------------
Good idea to check spots not as likely light exposed (including behind any labels).
[Edited on 8-7-2019 by AJKOER] |
yes it works without the :
I checked the bottom of the bottle and there are blisters on it perhaps fewer than the sides. The bottle is not opaque so some light would reach the
base but it would be significantly weaker particularly in the UV.
There are also blisters under the label even in the dark printed areas.
I will have to transfer the acid to a glass bottle soon if not now.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48temp9  |
I checked the bottom of the bottle and there are blisters on it perhaps fewer than the sides. The bottle is not opaque so some light would reach the
base but it would be significantly weaker particularly in the UV.....
|
For those who want to do a project using some statistics (assuming there are a good number of bubbles to proceed), one scheme is to divide the
bottle's surface area into blocks by drawing say blue lines to create equal area sectors for reduce light regions vs. more illuminated areas where the
sectors (basically pieces of pie for a circle for the bottom of the bottle and rectangles for the sides). Compute the area of respective sectors and
count the # of bubbles. Then compute the density of bubbles per unit area. To increase sample size, reduce the area of sectors, or easier, but not as
independent, rotate redrawing lines using a black pen (most facile if you do a rotation and maintaining area of respective new sample sectors,
otherwise since we are calculating density, a ratio estimate, the average of all sector densities for say light sectors likely moves, to a limited
extent, as well).
I would then apply a t-test for differences in mean density of bubbles for light and dark sectors. In the case of rotated data, exam the data in a
regression format and apply a first order auto-correlation correction (as derived from regression residual point errors).
Repeat the t-test by applying, say a log transform of the data (or other transformation per a Box-Cox Analysis of the data, to suggest the best
transformation to be consistent with Normal error distribution).
One could also record large bubbles only per sector (I suspect that large bubbles may indicate many local chemical bond failures, so possibly a
stronger indicator) and small bubbles only. Display statistical results for all data variations.
One could also randomly draw circles (label the circle with a #) of equal area (perhaps some overlap) on the bottle and perform the same analysis. A
related statistical approach is to randomly select circles by number (note: some circles may be randomly selected more than others, in this numerical
resampling technique) to construct the bubble density sample set, for expected high and low regions separately. Perhaps a better random approach to
'drawing circles' with a fixed area/diameter is to select two numbers, one in the range for acceptable points for an x-axis, and likewise for the
y-axis, for the center co-ordinates of possible circles.
For advanced students, get a text on spatial statistics.
[Edited on 9-7-2019 by AJKOER]
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Yes your link confirms the degradation of the plastic but it does not help understand how the blisters form.
My plastic bottle is still strong and its not possible to compress the blisters significantly with a finger. So the blisters are strong and/or under
significant pressure.
Perhaps they are caused by osmotic pressure of the diffusing water vapor from the outside of the bottle to dilute the HCl diffusing from the inside.
Perhaps a bubble in the plastic or something originates the blister. It would require significant pressure to start the bubble/blister but once its
formed much less pressure.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Here is a source https://www.nature.com/news/2007/070321/full/news070319-8.ht... but not much detail, but perhaps add electricity to the list of possible suspects,
as heat (as mentioned by wg48temp9), in addition to light and pressure, have been cited. To quote:
"chemists have succeeded in literally ripping the bonds between atoms apart, rather than using the usual suspects of heat, pressure, light or
electricity to drive a chemical reaction."
Some interesting comments out of Wikipedia (https://en.wikipedia.org/wiki/Electrostatic_discharge ) on ESD to quote:
"charged regions on the surfaces of styrofoam cups or bags can induce potential on nearby ESD sensitive components via electrostatic induction and an
ESD event may occur if the component is touched with a metallic tool....ESD can also be caused by energetic charged particles impinging on an object.
This causes increasing surface and deep charging."
So, in our particular case, if a bottle is subject to significant air flow, especially rich in dust, charged regions could form on the bottle's
surface, and perhaps even a deep charge into the HCl solution itself (which could release small amounts of hydrogen and chlorine atoms to attack the
plastic). So, anyone charged up about the possible idea of an electrostatic based charge effect on the bottles' polymer?
This case becomes stronger if there is observed a regional concentration of large bubbles on the bottle's surface, like the front versus the back of
the bottle. This also would be the case with a direct light effect (as opposed to diffused light).
Also, in our case of interest, could the very fluid internal to the bottle, together with the presence of sound waves, be the source of bond breakage?
'Sounds' far stretched, but in the one case of a specially designed polymer apparently yes, to quote from the first cited source:
"First, the sound waves create a flow in the solution that untangles each polymer string, beginning at one end. This provides a tugging force as one
end of the polymer is pulled. Second, the ultrasound creates bubbles in solution many times bigger than the polymer molecules. As these bubbles burst,
they both speed up the flow and the untangling of the polymers, and add a bang of mechanical energy".
Also per another source (see https://www.ias.ac.in/article/fulltext/reso/019/09/0821-0833 ) some possibly pertinent comments, to quote:
"polymers exhibit both elastic and viscous properties simultaneously and are viscoelastic. So, when a polymer is vibrated, part of the energy is
stored (elastic) and part is dissipated as heat (viscous) within the polymer."
And, of direct interest, a comment on fatigue life due to the damping properties of polymers, to quote:
"damping is the most sensitive indicator of molecular transitions and structural heterogeneities in polymers (plastics and rubbers). Many mechanical
properties such as fatigue life, toughness, wear and coefficient of friction are intimately related to damping."
Supportive of a sound effect would be a very uniform spread of bubbles on the bottle's surface, as could be the case with a thermal exposure.
Some mentions of mechanics leading to breakage, to quote:
"the researchers report in Nature1. In heat- or light-triggered reactions, the energy provided allows bonds to break, but there is no directional
element to the reaction....In this case, though, the tugging of the polymer strand means that the molecule is being stretched out. "We have a force
that's applied in a specific direction," says Moore. This helps to determine the shape of the final, broken product."
If I have to rate the possible suspects in declining order, my take is: heat, light, sound and ESD. The mechanics appear to be related to stretching,
hence, my number one favorite of heat.
[Edited on 11-7-2019 by AJKOER]
|
|
|
CouchHatter
Hazard to Others
  
Posts: 146
Registered: 28-10-2017
Location: yes
Member Is Offline
|
|
A little more than a year on, I have put my conc. HCl into a glass wine jug. It has worked exceptionally well, except that it has formed some deposit
on the rim of the glass. At first I thought it was silicone grease - it looks and feels identical. But the substance turned out to be water soluble,
so I ruled that out completely. Doesn't really narrow it down.
The jug is stored in a cabinet with several other acids, and the cap is in excellent condition and shows no signs of deterioration. Do you have any
idea what this is? Any tests I could do with such a small amount?
 
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
If HCl degraded silicone grease, it could be water soluble.
It clearly isn't HCl or water, so the real question is where the contaminant came from.
That should give a clue as to what it is.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I have a 30L canister half full of HCl and I just a month ago scavenged a few dL of it and it is as good as new. I suspect the materials aren't
actually HDPE, but some blend of (recycled) plastics, and possibly contain porosities too due to that, hence bubbling and failure.
If you use ordinary mason jars, bottles or stuff for storing anything that could attack the rubber, silicone or other secondary material of the bottle
top seal, cut a piece of PE plastic between the lid and the rim of the bottle. Even a piece from ziploc bag works exceptionally well. Some stuff will
just eat through the cap gasket and the cap itself in no time. In a hurry, I've just put an entire ziploc bag on top of a jar and closed it. The cap
seals the plastic to the rim and the contents can only reach either glass or PE.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Has anyone ruled out the use of CaCO3 as a cheap filler in plastics?
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
unionized,
That is a common filler in plastic 
One would hope that it isn't in plastic used for jugs of HCl
|
|
|