EvlRenne
Harmless

Posts: 39
Registered: 6-10-2018
Member Is Offline
|
|
Tryptamine question
Hello everyone,
I am not sure that I choose correct section, should it be organic or technochemistry, anyway.
I am interesting in synthesis of tryptamine and I like to challenge myself.
I've read a lot about thermal decarboxylation in high temperature boiling solvents and it is well studied and known path.
One day I was thinking about electrolytic decarboxylation, but, to be honest nothing even theoretical came to my humanists brain and at this point I
was thinking about photochemistry.
I started to dig in into the web, and not-surprisingly, found almost nothing, but the little light of hope still here.
I found couple of articles regarding photodecarboxylation of amino acids (see attachments).
And from that moment I have some question to professional chemists:
1) Do you think it will be possible to do photodecarboxylation of tryptophan?
2) What kind of condition should take place (including hv, solvent, catalyst, temp., reaction time)
Of course I am not worry about yields, I am interesting in science behind it.
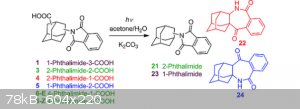
[Edited on 16-6-2019 by EvlRenne]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I don't see any attachments..
|
|
|
EvlRenne
Harmless

Posts: 39
Registered: 6-10-2018
Member Is Offline
|
|
That's weird, my reference was on this article: https://onlinelibrary.wiley.com/doi/abs/10.1002/ejoc.2016004...
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Decarboxylation of tryptophan is very easy. You dont have to bother with photodecarboxylation or anything like that.
Decarboxylation is accomplished by mixing about 80 g tryptophan in 250 mL of high-boiling solvent (xylene, DMSO, cyclohexanol, etc.), adding a dash of
a ketone (I like 5 g of cyclohexanone, but a couple grams of MEK works reasonably well), heat it to around 150 deg, and when evolution of CO2
ceases/solution is clear, the reaction is complete. This takes anywhere from 1.5 to 4 hours. After this is over, the solvent is boiled off (or at
least greatly reduced in volume), and the residue is dissolved in DCM. This is washed with a 5% NaHCO3 solution, then a distilled water solution, then
the DCM layer is separated off, dried with MgSO4, and the DCM is boiled off. You now have reasonably pure tryptamine.
Taken from Erowid
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
If you want an interesting project along those lines you could always try making monomethyltryptamine from calycanthine.
Grow some sweetshrub, extract the alkaloid, thermally decompose it, purify the resultant material.
|
|
|
EvlRenne
Harmless

Posts: 39
Registered: 6-10-2018
Member Is Offline
|
|
Quote: Originally posted by mackolol  | Decarboxylation of tryptophan is very easy. You dont have to bother with photodecarboxylation or anything like that.
Decarboxylation is accomplished by mixing about 80 g tryptophan in 250 mL of high-boiling solvent (xylene, DMSO, cyclohexanol, etc.), adding a dash of
a ketone (I like 5 g of cyclohexanone, but a couple grams of MEK works reasonably well), heat it to around 150 deg, and when evolution of CO2
ceases/solution is clear, the reaction is complete. This takes anywhere from 1.5 to 4 hours. After this is over, the solvent is boiled off (or at
least greatly reduced in volume), and the residue is dissolved in DCM. This is washed with a 5% NaHCO3 solution, then a distilled water solution, then
the DCM layer is separated off, dried with MgSO4, and the DCM is boiled off. You now have reasonably pure tryptamine.
Taken from Erowid |
Thank you for your reference, but the reason I am trying to find some information, because traditional way is all know and easy to do, what is not
interesting for me, and as I mentioned before, I am not looking for height yielding procedure, I am looking for interesting project to do.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
It is promising if you have the courage and the will to walk unwalked paths. You may found something interesting.
However, the reaction you are pursuing is not too amateur friendly. Not as if the decarboxylation reaction would not be amateur friendly but you would
need good analytical capabilities to map the products. Someone wrote somewhere that the main difference between an amateur lab and a professional one
is in the analyzing capabilities. Which I found very true.
You may get tryptamine if you solarize a flask of tryptophane in acetone e.g., but you will surely get a lot of other things as well and you won't be
able to tell how much tryptamine you got (if at all) without the analysis!
Electrochemical way? Hm, I think reduction of the carboxyl group may be possible, maybe dimerisation would also be possible (Kolbe electro synth),
decarboxylation: I don't know.
Again: how would you analyze the broth in the end?
I don't expect anything would miraculously crystallize from the "soup" and even if it had: how would you identify those crystals?
This is the problem of original research in an amateur setting!
What if you tried to modify one, proven, working method slightly at first and see what happens? Then modify it further until you either get nothing or
a near theoretical yield! 
|
|
|
|