| Pages:
1
2
3
4
5
6
..
13 |
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
I get what you mean. Im still trying to find a freaking flower pot for electrolytic H2SO4 production  It pisses me off cause everytime I try it, SOMETHING has to go wrong, but everything else goes right!!! If only
you could combine all the rights and toss out the wrongs It pisses me off cause everytime I try it, SOMETHING has to go wrong, but everything else goes right!!! If only
you could combine all the rights and toss out the wrongs 
Keep up the optimistic attitude 
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Well, this is the first time someone has called me an optimist, but there might be some merit to the designation....
I have a new idea about the clay. As I see it, the main problem is that is shrinks as it dries.
So --- maybe, just maybe, if one where to replace the water with a solvent that evaporates very quickly (like e.g. acetone), the clay wouldn't
shrink but be full of small air voids instead.
It's worth a shot.
Btw, what kind of flower pot are you looking for?
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
A flower pot that is unglazed and thin enough to use for a salt bridge.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
| Quote: |
A flower pot that is unglazed and thin enough to use for a salt bridge.
|
That's going to be hard to find  . .
If you don't mind the labor, you could buy one that is too thick and sand it down to an appropriate thickness. The process will consume sandpaper
at an alarming rate though......
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
My god! Thats just crazy  Guess I'll never get my salt bridge Guess I'll never get my salt bridge 
Edit: Btw, what would be the max thickness where it could still work?
[Edited on 3-3-2004 by Saerynide]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
I'm sorry, I have absolutely no idea how thin it has to be. I guess it depends on what type of clay was used, and which temperature was used when
firing it.
But you shouldn't have to sand down the entire pot. A 10x10cm area would suffice, no?
And you could use one of those machines with sandpaper on that vibrates. That would save you the excercise of doing it by hand.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Axehandle,
I know you are working in sulfuric acid production, but how is BE reactor. This is a good project and there are no news in you site.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Well, not being a female, I have no simultaneous capacity and the SO3 reactor takes up all the time I've got. The burner is almost done, and then
there's basically only the heater left.
I intend to continue the B-E reactor after the sulfuric plant is done. I have some improvements in mind, namely a static arc drawn out in an oval
shape by magnets (if I can find full-wave rectifiers rated for 10kV, 60mA...). I'll cast the outer lightning ring in brass. It will be
water-cooled. The inner electrode (a rod) will be consumed over time and must be constructed so it can be fed down. Did that make any sense?
I'm still evaluating materials. Gee, I didn't know material science was this exciting. Heat capacity, ease of work, conductivity... etc.
I'm actually feeling a bit stressed out at the moment.

My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
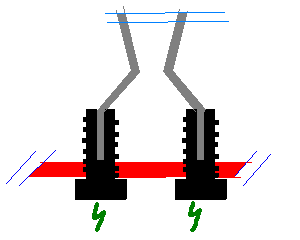
Testing a concept --- here we go
OK. Since I could never find a good way to mount electrodes in my old reactor contraption, I kind of gave up. Until now, that is. Sometimes ideas come
to me in my sleep, and that was happened today.
The new electrode idea is very simple, much simpler than the old one -- and as many would probably agree; simple ideas are the hardest to come up with
but tend to work best.
It struck me: Why not use simple disposable electrodes that can be replaced as they are burnt up? Made of simple, always available 2mm steel wire that
is easily bent into shape? Why not? Why not take that one step further, shall we:
Two simple bolts, interdistance around 20mm, protrude from the bottom into the reactor chamber. The bolts both have 2mm holes drilled lengthwise
halfway through them. The disposable electrodes are simply stuck down into the holes and bent to shape. The very small intersection area of the wire
will also insulate the electrode holders (the bolts), and thus the reactor bottom, from the immense heat building up in the electrode wire, or so I
hope. If not, using very much larger bolts should help. I'm attaching a very simple drawing.
Proof of concept using 2mm mild steel wire as discharge electrodes:
The test mount (I have 1.5mm, 3mm, 4mm, upto 17mm drill bits but couldn't find any 2mm ones so I had to tie the electrodes in place using 0.5mm
steel wire...):

I ran this contraption for a 2..3 minute run after having wetted the inside of the glass jar with water to become nitric acid. Lacking (unable to find
more like it) blue litmus paper I sacrified my tongue in the interest of science. I think I nitrated it -- it was the most foul, sour, evil stuff
I've ever tasted and it still hurts. I was still pleased though... The steel wire showed no sign of turning red hot, although it got very hot as
my hand found out touching it by mistake...
Tomorrow I'm getting some 2mm drill bits and a threaded rod (will be easier to drill in than nuts). I'm back in business!!!!!!!!!

My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Very nice work. I continue to enjoy your work and pictures. Would you consider selling tickets for a tour of your lab?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Nice! Good to see you're gonna finish it 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
I am waiting most eagerly to see your work finished. remember to update your
site with your new "findings". remember to update your
site with your new "findings".
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Aaargghh! No pressure, no pressure...
Edit1: Holes drilled. I actually came up with an improvement: The holes will be slightly (1mm more) larger than the electrodes, and tha latter will be
kept in place by set screws going into the bolts perpendically. That it if I can find an M3 threading bit... I know I have one around here
somewhere... might have to use a thicker threaded rod as well. Ah, it's all coming back to me.
BTW, what are those threading things for making threaded holes ("gängtappar" for any Swedes here) called in English? I know their opposite
sex is called "dies", at least I think so.
Edit2: Thinking that it would be much easier to work with aluminium threaded rod (or copper, or brass -- still, Al should be the most resistant to NO2
+ gaseous HNO3). I'll have to thread a round bar myself ofcourse. Hmm, can't get any until Monday, but I can use my freshly drilled steel
nuts to experiment with until then. Have to see what temperature the bolts and the vessel get to, if it's too high I'll have to employ two
identical reactors in series so they only get ~1/2 as hot (simple enough using 5 litre jam jars as I am). It's certainly not borosilicate glass,
heh...
[Edited on 2004-8-21 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
One makes threaded holes with a tap. Taper, plug, and bottoming taps. Nice madproject.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Taps it is, then. 
Just did a 10 minute test run, taking pictures of the air inside the reactor getting browner and browner with NO<SUB>2</SUB>:
http://species8472.dyndns.org/no2/testing.html
Note that the vessel is hemetically sealed: Wouldn't want all that nasty NO<SUB>2</SUB> out in my living room...
[Edited on 2004-8-21 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
Very nice axe. Just a thought:
What if one did this experiment under a predetermined pressure in a pressurised vessel. Wouldn't the pressure decrease as NO2 was formed?
If so, the pressure could be maintained by a feed from an air compressor. If all goes according to my 'theory' the NO2 would form HNO3 with
any water present in the vessel and condense due to the pressure. This could then be collected at an outlet valve at the bottom of the vessel.
I suppose there could be an explosion risk involved.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Why keep the vessel pressurized? It won't save time, the air throughput will be the same, right, although replenished more infrequently? Although
I can imagine that the reaction would be more efficient with rising pressure, I don't know -- perhaps someone does?
Still, I'd guess the absorption of the NO<SUB>2</SUB> would be more efficient at higher pressurizes, but this is a guess also.
My plan for absorption is bubbling the output air + NO<SUB>2</SUB> mix through water --- keeping water inside the reactor would require
cooling of the same; it gets as hot as a lightbulb, not surprising when using a 540W arc inside a glass jar  . .
Edit1: Another note -- mounting plasma discharge electrodes inside a glass jar is very non-trivial. I gave up on the problem and it took me half a
year to solve the problem (could be me being stupic rather than the problem being hard though), and that's without allowing water condensing in
the reactor bottom. Even supposedly de-ionized water gets somewhat conductive when used with voltages in the 10kV range, stopping the formation of any
arc. I had many woes with different sulutions.
[Edited on 2004-8-21 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
What is the exact mechanism for NOx formation?
Is it formation of nascent oxygen followed by attack on molecular dioxygen to form ozone and its subsequent action on nitrogen, or is it the action of
the nascent oxygen on the dinitrogen, or is it the action of electrical discharge on dinitrogen to form nascent nitrogen and its action on dioxygen,
or some combination thereof?
I'm asking this because weather you should run this under pressure or not is the same kind of equilibrium shift that is involved in any reaction.
If the reaction produces more mols of gas then the initial products it would be good to run it under reduced pressure, if it produces less mols then
it would be good to run under pressure.
Personally I like the idea of a constant stream of atmosphere being lead into the reactor and an exit tube running though a bubbler into water and
that exit tube running doing other things but finally ending in a vacuum pump to keep things flowing in the direction necessary to keep dissolution of
the products.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
From the little information I've found regarding the physics of this reaction, it's hard to deduce exactly what is happening. All sources
cite the formation of nitrogen monoxide NO, which at room temp quite immediately graps another oxygen like
2NO + O<SUB>2</SUB> <--> 2NO<SUB>2</SUB>
The few (very few, like 2) sources regarding this reaction I've seen claim that N<SUB>2</SUB> <i>and</i>
O<SUB>2</SUB> are both split into free atoms 2N + 2O at plasma temperatures, but whether its really true or if it's only one of
{N<SUB>2</SUB>, O<SUB>2</SUB>} being split, and in that case which one, I don't know.
It seems to work anyhow, but I'd very much want to know exactly what's going on.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Ho-ho, those pictures were certainly very good. Very nice to see the brown gas form. You just need to add some sort of a valve to it to let the
nitrogen dioxide out, cool it to form the dimer(easier to handle that way), and at the same time give the reactor fresh air to react with.
By the way, the formation of NOx gases is truly puzzling. For example, the catalytic oxidation of ammonia using chromium (III) oxide is a bit odd.
Refer to http://www.chem.leeds.ac.uk/delights/texts/expt_14.html
When the reaction is carried out in a open vessel, mostly nitrogen gas is formed. but if the reaction is carried out in an enclosed jar, nitrogen
dioxide is formed.
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
maxke
Harmless

Posts: 7
Registered: 18-5-2004
Member Is Offline
Mood: No Mood
|
|
I think a setup with a compressor will still benefit a setup like the one shown on your site axehandle.
compressor <-> pressure barrel (3bar controlled) <-> valve 1 <-> arc chamber <-> valve 2 <-> water absorption 1
<-> water absorption 2
My reasoning : if you want to keep no2 from releasing into your room you need to push it away somehow , to the absorption vessel.
So if after 10 minutes a batch of NO2 is ready , valve 1 and valve 2 are opened so to push the NO2 into the absortion and to purge all or almost all
NO2 in the arc chamber with somewhat pressurized air.
I would setup the compressor so that it turns on when the pressure drops under 2.5 bar and turns off when the pressure is greater than 3 bar. (this
requires a pressure sensor and shmitt trigger setup/ or any other setup that does the same)
So if you'd like to fully automate the process , you could also set the valves to open / close at set intervals , and also you could modify your
absorption vessels to purge fully absorbed NO2 / HNO3 after 60 minutes ... all this for the sake of amateur rocket science right ??
What do you think ?
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
I don't know. A couple of places I've read that formation of NO<SUB>2</SUB> gets faster as the concentration of same goes up, up
to a point ofcourse.
I suspect the only way to find out whether "batch" air replacement of "continous" air replacement is optimal is to try them both,
which is simple enough using a timer and an air pump.
I like the high pressure idea, but I'm trying to keep the design as simple as possible -- which would somewhat be thwarted by having to design a
vessel that can stand positive inside pressures in the 2..3 bar range. Worth lots of thinking though...
I've added a couple of pictures of what the electrodes looked like after having been left in NO<SUB>2</SUB> overnight at the bottom
of this log:
http://species8472.dyndns.org/no2/testing.html
[Edited on 2004-8-22 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
Another problematic factor you mentioned axe is temperature control. The vessel would then have to be placed in a water jacket to keep it cool which
will only add to the complication.
I suppose the best is to try it which I'm keen to do once I've acquired a HV source.
I was also wondering if it is possible to mix the NO2 with water vapour hoping the NO2 would be absorbed by the water in vapour phase, being aided by
the pressure. This would mean that the overall volume of gasses would decrease hence lowering the pressure and as mentioned above a feedback triggers
the compressor to reinstate a pressurised environment.
The water vapour could be ensured by some kind of variable humidifying setup between the compressor and the pressure vessel. It may be true that the
concentration of HNO3 collected can be controlled by adjusting the humidifier.
The pressure vessel would probably need to consist of 2 parts, the reactor itself where temperatures are relatively higher, and a condensor, where
temperatures are forced to cool. This is where, if my theory 'holds water', the HNO3 is collected using a timed solenoid or a simple ball
valve. The design of the vessel would have to be such that these conditions are optimised. One would have to design the outlet in order to prevent the
the acid from spraying everywhere as a result of the pressure.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Is it possible to use a induction coil instead of NST transformer? As i have no access to such transformers, is it alright if i used other sources of
plasma discharge?
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Saerynide, why not make your own unglazed clay pot? Raku clay is very porous to begin with, and if you add grog (bits of prefired and ground up clay)
the clay will get even more porous.
Raku clay is cheap. 10 dollars for 25 pounds.
It has high thermal shock resistance and can be fired quickly at low temperatures- think bonfire. 
With this method you could form nearly any kind or shape of reaction vessel you wanted.
It could say "made by Saerynide industries." on the bottom. A definite bonus. When people ask where you got it, just point to the label.

Then again, it is just another thing that can go wrong.
|
|
|
| Pages:
1
2
3
4
5
6
..
13 |