ZetekitoxinAB
Harmless

Posts: 19
Registered: 31-7-2019
Member Is Offline
Mood: Omberacetam
|
|
Vinconate identification
So I have recently rediscovered an old bottle of a substance, which I bought many years ago from a nootropics vendor, on ebay. It was advertised as
vinpocetine and I remember it was very cheap, but he had good reviews so I decided to purchase it. At that time I had access to a chemistry lab and
after taking it as vinpocetine (about 30-40 mg per day) for some time without any apparent side effects, I have decided to take a melting point. It
turned out to be 119-121 °C, which was different from the literature value of real vinpocetine of 147-153 °C. Substance was pure according to TLC
and I was able to send it, along some other chemicals, to an ESI-MS analysis which gave a Molecular weight of 296. Unfortunately I could not obtain a
more advanced NMR spectra. Now I have moved away and dont have too many possibilities for quality analysis; I just have a few general reagents. I have
reckoned after some web search that the slightly pale yellow crystalline substance was in fact vinconate - a synthetic chemical with purported
nootropic effects but otherwise very obscure - likely substituted by the original trader for profit. The melting point and M do correspond to
literature values of vinconate. I think that these circumstances strongly indicate that in fact it is vinconate, however I cant get over the fact that
the substance is completely insoluble in 33% HCl, even after boiling. That is very strange in my opinion because of the supposed strongly basic
tertiary N; even if synthetic it resembles a classical indole alkaloid. I mean even strychnine, which has a larger molecule and a similar basic N,
form highly soluble salts with various acids. The substance is soluble in acetone giving a light yellow solution, which reacts with K dichromate in
dilute sulfuric acid but not with iodine. Interestingly enough, I didnt find any reference to hydrochloride salts of vinconate or vinpocetine, instead
they are sold as free bases. I would be really interested in possible explanations regarding the insolubility in conc. HCl and the lack of reaction
with iodine, even though it has an isolated double C=C bound.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Electrophilic addition proceeds via a carbocation and carbocations cannot form next to an electron withdrawing group (as ive had
pointed out to me).
An ester like the one seen in Vinconate is an electron withdrawing group and thus its not possible for the alpha carbon next to it to undergo
electrophilic addition with iodine or any other halogens or hydrogen halides.
As for you solubility issue, you mention boiling it in conc HCl, i would assume under these conditions you would hydrolyze the ester producing a
carboxylic acid. As the equilibrium lies quite heavily in favor of the carboxylic acid.
Perhaps you should instead try to add a stoichiometric quantity of HCl and then filter off any precipitate, then weigh the precipitate and titrate the
filtrate.
If you get nearly the same amount of solid back out of solution as you started with then the filtrate should contain very little or practically no
HCl, this would indicate that the hydrochloride salt has formed.
If you instead manage to dissolve the solid with dilute acid then well you've answered your question.
It should also be noted that not all protonated amines are necessarily highly soluble in H2O, the shier organic bulk of the compound may hinder its
solubility by having a rather non polar end.
And to answer a question you may also pose, not sure if your already aware.
The indole amine is not basic, this is due to that alkene next to it which prevents the lone pair of electrons on the nitrogen from being available to
accept a proton.
There is only one amine available for protonation on Vinconate.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by Assured Fish  |
An ester like the one seen in Vinconate is an electron withdrawing group and thus its not possible for the alpha carbon next to it to undergo
electrophilic addition with iodine or any other halogens or hydrogen halides. |
This is not true. The addition of halogens proceeds through an enol-like intermediate. See for example: https://en.wikipedia.org/wiki/Hell%E2%80%93Volhard%E2%80%93Z...
Edit: and for vinconate in particular, it wouldn't even be an alpha-halogenation, but rather addition of the iodine across the double bond. However,
iodine is often quite slow to react with alkenes, especially (as you mentioned) when there's an electron-withdrawing group. I expect that bromine or
chlorine would react, however.
[Edited on 2019-8-2 by Metacelsus]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
The Hell–Volhard–Zelinsky halogenation is not the same reaction that was attempted by ZetekitoxinAB.
He used straight elemental iodine, thus he was attempting an electrophilic addition reaction.
https://en.wikipedia.org/wiki/Electrophilic_addition
The reaction you are describing is also initiated by enolation of the carbonyl, meaning the alpha carbon is left with a free electron without needing
be become a carbocation for the neucleophile to then pick up.
Take a look at both mechanisms.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
ZetekitoxinAB
Harmless

Posts: 19
Registered: 31-7-2019
Member Is Offline
Mood: Omberacetam
|
|
Thank you for your insights ! I have tried again dissolving the substance in acid: 1 mmol (0.29 g) was suspended in 10 ml cold water and 0.2 g (slight
excess) 33% HCl were added. No modification was observed whatsoever, the quantity of the suspension seems visually the same I will filter it tomorrow
and weight it.
I have also tried the reaction with bromine, as suggested: in a 20 ml flask equal volumes of hydrogen peroxide 3% and sulfuric acid 37,5% were mixed
giving about 15 ml solution. To this a small quantity (less than 0.5g) KBr was added, the solution turned almost instantly yellow with a strong
bromine odor. 10 ml dichlormethane were added and the mixture was stirred, giving the organic layer an orange color. In a separate flask, rinsed and
dried previously with acetone, 0,3 g test substance and 10 ml DCM were added giving a clear yellow solution.
I took the bromine containing DCM with a pipette and added it to the test DCM solution. Decoloration was instant but interestingly it also started
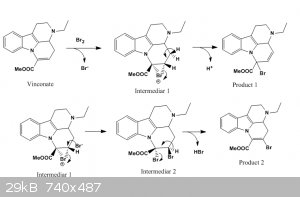
slightly fuming (HBr). I would say that if the substance is vinconate (which is highly likely as both M and melting point correspond), there was an
addition of bromine to the isolated double C=C bond, followed by an elimination reaction. I have attached a possible mechanism; I would presume that
the bromonium intermediate would more likely react with another bromide giving the dibromo derivate which would eliminate HBr giving product 2.
Here is a synthesis published for vinconate: https://www.eosmedchem.com/article/83.html
Condensation of ethyltryptamine with VINC 006 is very nice, but the structure of the intermediates until VINC 006 is very dubious. Maybe the other
carboxylate group is selectively cleaved, the other esther group reduced with NaBH4 to the primary alcohol which is oxidized with PCC to the aldehyde.
The synthesis just seems overly complicated with many steps; I am sure VINC 006 could be made on an easier route.
In any case, I have started to think if the insolubility of the stuff has anything to do with the spatial structure of the molecule, where the lone
electron pair interacts with the esther conjugated double bond, lowering basicity.
Also very likely vinconate is a racemate, as suggested by pubmed; but pubmed also gives reference to HCl salts. Maybe these salt could be obtained by
combining the drug with dry HCl in a solvent, who knows...
|
|
|
|