Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
2,2'-Bis-imidazole
As part of my ongoing investigation into the complex formation of bis-heterocyclics with the N=C-C=N motif like bipyridyl and 1,10-phenanthroline I
have tried to prepare the title compound. This compound was first prepared by Debus 1) by the reaction of glyoxal solution with aqueous ammonia and
the reaction and its products was again studied by Lehmstedt 2). The reaction is simple to carry out and the sparingly soluble bis-imidazole
crystallises out almost immediately, unfortunately the reaction is messy and the yield of bis-imidazole is poor (my yield 0.17g from 5g of glyoxal
(10ml of 40% solution) c 4%) though with care about 1.2g of monomeric imidazole can be recovered from the residue.
Debus realised that 3 molecules of glyoxal reacted with 4 of ammonia to give the bis-imidazole but the production of imidazole was rather curious as
it required a single carbon unit. Debus investigated the residue and found that formic acid was present. This suggests to me that under the alkaline
condition part of the glyoxal undergoes a sort of Cannizzaro type reaction cleaving formic acid from the bridging glyoxal unit before it could react
with more ammonia.
Do the more theoretically adept agree with my idea and if so my question is then how to reduce the effective alkalinity of the ammonia? I tried
running the reaction in acetic acid with ammonium acetate, this is not without precedence since it is the basic of the synthesis of numerous imidazole
derivatives using a dione (eg Benzil) + ammonium acetate + an aldehyde in acetic acid. The reaction work with glyoxal and formaldehyde to give
imidazole but in poor yield. But it doesn't seem to work with glyoxal as both dione and aldehyde or at leat the dark crude oil like reaction mixture
doesn't seem to have any precipitate in it (bis-imidazole is very insoluble).
Do you think I could run it in water with ammonia solution and an ammonium salt to suppress the protonation of the ammonia and hence limit the OH- ion
production via the common ion effect? Is there an alternative proxy for ammonia in neutral conditions?
I am going to try a run buffered with ammonium chloride.
1) J. L. Ann. der Chem. 1858 p199-208
2) J. L. Ann. der Chem. 1927 p253-275
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
With a bit of assistance from a US patent 2003/0199700 I have succeeded in preparing crude 2,2-bis-imidazole. I am still working up the product which
is rather brown. Basically my idea of buffering the system with an ammonium salt is the way forward. In the patent they use ammonium acetate as the
sole source of ammonia and the yield of the bis-imidazole is much better at close to 50% and about 30% recovery of imidazole from the filtrate by
precipitating the latter with oxalic acid.
When I have completed the work-up of the crude products I will post a full description of my procedure.
Imidazole and bis-imidazoles are amphoteric but the nitrated equivalents are fairly acidic as well as acting as ligands with metallic ions. Should be
interesting.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
A query: Why does ammonia react with glyoxal to produce five membered heterocyclics while formamide reacts with glyoxal to give a six membered ring?
The reason I ask this here is that during the work-up and purification of the 2,2'-bis-imidazole and the imidazole oxalate from the above preparation
I have recovered a third compound. It forms characateristic short, colourless, glassy prisms that are sparingly soluble in boiling water and
practically insoluble in cold water. Its solubility does not increase in dilute HCl (<0.5M) though it increases slightly in stronger HCl (>/=
5M) which distinguishes it from bis-imidazole which is freely soluble in very dilute HCl(<0.1M) and can be isolated as a hydrochloride salt. It is
not precipitated by oxalic acid and it is not noticably more soluble in dilute NaOH (1M) solution thus distinguishing it from imidazole and imidazole
oxalate.
I wondered if this compound might be a six membered ring, basically the same as the formamide-glyoxal condensation product (See Axt's prebublication
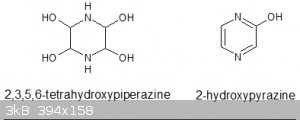
article on TEX) less the two formyl groups: So 2,3,5,6-tetrahydroxypiperazine or possibly its aromatized-by-dehydration product 2-hydroxypyrazine. Is
this likely?
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
This is interesting work, post some pictures if you get a chance. As far as forming a condensation product, what are your reaction conditions? Would
it be possible to hook up a dean stark apparatus to your reaction to measure water produced? That might help with figuring out your stoichiometry.
Another thing which you might be able to do is measure your boiling point elevation/freezing point depression to determine if you are dealing with a
pure compound or a salt of some kind. It sounds like it it is a pure compound, so if it is, see if you can't determine a melting point/freezing point.
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Steam; thanks for the comments, I am currently producing a write-up on this procedure since there doesn't appear to be a modern and worthwhile
procedure in the literature.
As to using a Dean and Stark type set-up, the problem is that the reaction is conducted in an aqueous medium simply because the glyoxal comes as an
aqueous solution.
I was rather wondering if someone out there in the greater SM membership that could shed some light on what determines whether you get a 5 or 6 member
ring.
I am going to try and oxidize the compound to see if I can produce a polyhydroxypyrazine. There is an interesting precidence for this in that if the
formamide is replaced with nitromethane the resulting 1,4-dinitro-tetrahydroxycyclohexane can be converted easily into aromatic compounds by
dehydration and possibly also by oxidation.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The mp of mp of 2-hydropyrazine 185-190 °C, according to Aldrich, so easy enough to test that idea. I don't think the 2,3,5,6-tetrahydroxypiperazine
would be stable, if it formed at all, being a multiple hemiaminal.
The use of ammonium acetate to buffer the reaction seems good, you could try ammonium carbonate or chloride to see if the counter ions matter at all,
they will change the pH a bit.
Nice work, I did undergrad research in a lab that made some bi and tripyridyle ligands and complexes, back when they were more novel, I didn't work
in that area, but made other linked pyridyl ligands, mostly with carbonyl urea type linkers. Very polar molecules. But they did chelate copper,
Cobalt, and nickel, I seem to remember.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Dr.Bob I am interested in your comments about the stability of the postulated 2,3,5,6-tetrahydroxypiperazine. The N,N'-diformyl derivative is an
important intermediate in the preparation of the explosive TEX see: http://www.sciencemadness.org/talk/viewthread.php?tid=6000#p...
Do the formyl groups provide stability? I prepared some of the formyl derivative and am going to try and aromatize it. My first plan is to try
converting it to the tetra-acetate ester with acetic anhydride+H2SO4 and then treat this compound with pyridine (there is a precident for this
technique which I will find the ref. for). If this works then I plan to try turning my compound into a hexa(?) acetate ester and then aromatize it to
(hopefully) the same compound; 2-pyrazine acetate. I wonder if this compound will smell anything like 2-acetyl-pyrazine (roast chicken  ) )
I will post my full experimental details tomorrow in the prepub. section along with some pics of the products and the purification procedures.
I plan to try and convert the bisimidazole into Lehmstedt's tetranitro and dinitro bisimidazoles as seem toform salts/complexes too.
|
|
|